
|
||||||||||
|
||||||||||
| Favorite Tweets by @thaibirding | ||||||||||
|
||||||||||
 Buy Birds of Thailand on Amazon.co.uk |
||||||||||
| Site Map ; Contributors |
| A
nesting pair of Gecinulus woodpeckers in a likely zone of
intergradation between Pale-headed Woodpecker G. grantia
and Bamboo Woodpecker G. viridis By Philip D. Round, John M. Hobday, Rungsrit Kanjanavanit & James S. Steward |
|
| Tweet | |
| Note:
This article was originally published in Forktail 28 (2012) the journal
of the Oriental
Bird Club (OBC) and was kindly submitted by Philip
D. Round. Please support the OBC's conservation work by visiting the OBC website and becoming a member. |
|
| ABSTRACT A nesting of a pair of Gecinulus woodpeckers in a possible zone of intergradation between the parapatric taxa Pale-headed Woodpecker G. grantia and Bamboo Woodpecker G. viridis is described. While the male looked like a more or less typical G. viridis the female bore plumage characters that appeared intermediate between G. grantia and G. viridis. Additionally a specimen labelled as G. grantia indochinensis, collected in Thailand in 1964 (the only record for that country), also appeared atypical, showing characters somewhat intermediate between G. grantia and G. viridis. It is likely that a narrow hybrid zone between G. grantia and G. viridis exists where the two come into contact in northern Thailand and, presumably, northern Laos. Recommendations for further surveys are made in order to determine the extent of postulated hybridisation, and additionally to investigate the ecological and taxonomic relations of these two taxa. |
|
| INTRODUCTION Gecinulus woodpeckers are medium-sized, three-toed woodpeckers that occur in intimate association with large-culm bamboos. The five or six accepted taxa are either treated as constituting two allospecies (King et al. 1975, Robson 2008), or as one polytypic species (Short 1982, Dickinson 2003). If the former treatment is followed, two subspecies of G. viridis (Bamboo Woodpecker) are distributed in East and South Myanmar and most of Thailand (G. v. viridis), and Malaysia and adjacent southern Thai provinces (G. v. robinsoni). The (mostly) more northerly distributed G. grantia (Pale-headed Woodpecker) ranges along the Himalayas from eastern Nepal, north-east India, to (mainly north and west) Myanmar (nominate grantia); Fujian and Guangdong, south-east China (G. g. viridanus); Yunnan, Laos, marginally northern Thailand (a single record, mentioned below); and Vietnam, from Tonkin south to (probably south) Annam (G. g. indochinensis). A further subspecies, G. g. poilanei, described by Deignan (1950) from Cochinchina, southern Vietnam, is doubtfully distinguishable and was regarded as a synonym of indochinensis by Short (1982). Nowhere within this large, aggregated range of the various taxa is there indisputable evidence of sympatry between birds in the viridis and grantia species groups. We here report on a nesting pair of Gecinulus, observed in Chiang Rai province, northern Thailand, in which the female showed plumage characters intermediate between those of G. viridis and G. grantia. We were concerned to conduct a review of the distribution of both species where their ranges approach each other, and to determine whether there were any other indications that the taxa G. v. viridis and G. g. indochinensis might intergrade in their narrow zone of contact. |
|
| STUDY
AREA The field observations were made at Ban Saen Jai, Mae Fah Luang district, Chiang Rai province, 20°12’N 99°46’E, c.12 km westnorth- west of the town of Mae Jan, and some 65 km due west of the collection site of Thailand’s only G. g. indochinensis specimen. The habitat was farm and plantation in steep hilly country at c.600 m elevation. The area has long supported villages of the Akha, a Tibeto-Burman ethnic minority group of (traditionally) pioneer shifting cultivators, but in recent years large tracts have been bought by urban landowners. While most of the area is deforested, and planted with hill-rice and corn, a c.20 ha community forest, preserved according to Akha land-use tradition, lies adjacent to Ban Saen Jai village. Additionally, ribbons of secondary forest and bamboo along steep gullies (some spring-fed) maintain connectivity among wooded fragments in the otherwise near-totally deforested landscape. During the period of the study the afternoon temperature in the general surroundings varied between a low of 28°C in mid-March and a high of 37°C in mid-April. The temperature on the floor of the shaded, woody gullies was noticeably (c.2°C) cooler than that of the immediate surroundings. |
|
| METHODS Intermittent observations were made on a single nesting pair of Gecinulus at Ban Saen Jai, whenever one or more observers was present, during 10 March (when the nest was discovered) to 18 April (when the young fledged). Additionally we sought specimens and sight records of G. viridis and G. grantia in northern Thailand and northern Laos, focusing particularly on the details of the Lao range of G. grantia, since Laos is the only country other than Thailand where the ranges of G. grantia and G. viridis approach closely and, indeed, may overlap. We did not attempt any review of specimens from Myanmar where G. g. grantia is known from the south-west, west, centre and north, and G. v. viridis from the south and east (Robson 2008). It is not clear whether this apparent discontinuity in the distributions of the two in Myanmar is genuine or merely an artifact of sampling. While the Mekong River, some sections of which delineate the national boundary between Thailand and Laos, flows generally north to south, in places it also flows west to east (or even briefly south to north). In the context of this paper, the terms ‘east of the Mekong’ and ‘north of the Mekong’ can be used interchangeably, as can west/south of the Mekong. In discussion of specimens, the following abbreviations are used: BMNH Natural History Museum, Tring, UK; CTNRC Centre for Thai National Reference Collections, Bangkok; FMNH Field Museum, Chicago; MCZ Museum of Comparative Zoology, Harvard University; USNM National Museum of Natural History, Smithsonian Institution, Washington, D.C. |
|
| RESULTS Distribution and vocalisations Gecinulus grantia is found widely throughout northern, central and southern Laos, in both primary and degraded semi-evergreen, dry evergreen and mixed deciduous forest (Thewlis et al. 1998, Duckworth et al. 1999, Evans 2001). Three specimens from Bokeo province, at Lo-Tiao, c.20°28’N 100°22’E (Figure 1), comprise two males, MCZ 267140 and MCZ 267142 collected on 6 and 7 January 1939 respectively, and a female, MCZ 267141, collected on 6 January 1939 (Figure 7). Inexplicably, the account in Delacour & Greenway (1940) implies that only a single specimen (‘un exemplaire’) was collected at Lo-Tiao. A further male specimen was collected from Phongsaly province, probably Ban Khomen, Pongsaly district, at 21°39’N 102°08’E (Bangs & Van Tyne 1931), on 28 April 1929 (FMNH 78170). |
|
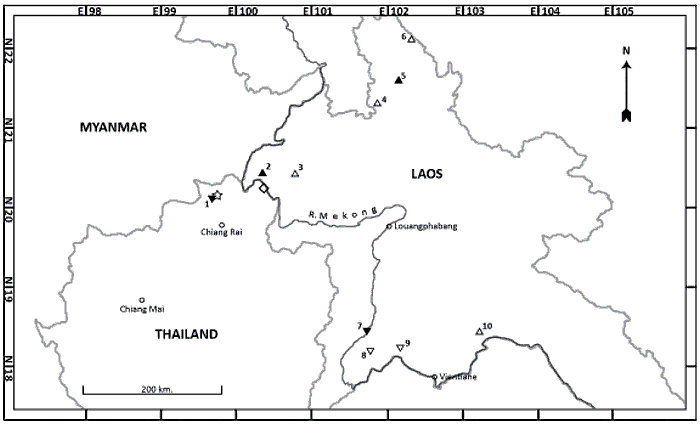 Figure 1. Map to show locations of specimens and sight records of Gecinulus woodpeckers in northern Laos and adjacent Chiang Rai province, northern Thailand. |
|
| Neither
specimens nor photographs are available for further reported G.
grantia in northern Laos, which consist of: one handled in
Nam Kan National Biodiversity Conservation Area (NBCA), Bokeo province,
probably c.20°28’N 100°48’E (Pasquet 1997);
sight records beside the Nam Mang in Phou Khaokhoay NBCA, Vientiane
province, c.18°31’N 103°12’E (Thewlis et
al. 1998); and in Phongsaly province at Phou Dendin NPA, c.22°09’N
102°22’E (identification recorded as provisional) and
at Ban Naten, 21°20’N 101°52’E (Fuchs et
al. 2007). The lack of any further records known to us probably
reflects the paucity of survey in much of northern Laos rather than
indicating a genuine scarcity there. The sole record of G. grantia for Thailand is a female specimen, USNM 534656, labelled G. grantia indochinensis, collected by B. King at Chiang Khong, Chiang Rai province (20°17.7’N 100°23.5’E) on the south (west) bank of the Mekong, where the river forms the national boundary, on 26 April 1964 (King 2007). Gecinulus viridis is widespread but uncommon in Thailand, in evergreen and deciduous forests where large-culm bamboos are present, up to an elevation of c.1,400 m (Lekagul & Round 1991). The only historical record of G. viridis from Laos is a specimen, BMNH 1955.1.2505, from Ban Moung Liap, on the Mekong River, Xaignabouli province, c.18°29’N 101°40’E (Robinson & Kloss 1931). Present-day Lao maps give the village name as Ban Muangliap while the name in today’s official government use is Ban Phaliap (J. W. Duckworth in litt.). As already discussed by Duckworth (1996), the basis for Delacour’s (1951) statement that the specimen probably came from the west bank (‘rive droite’) of the Mekong may have been nothing more substantial than the supposition that the east bank (‘rive gauche’) would support G. grantia indochinensis, presuming that the two species would be unlikely to occur together. Its origin in this respect should therefore be treated as uncertain. Recent surveys have, in fact, extended the Lao range of G. viridis south and east, the species having been widely found up to at least 20 km north of the north bank of the Mekong, in Sangthong district, west of Vientiane (several individuals, including pairs with young: Duckworth 1996). There is also an intervening record somewhat north-west of this, also well inland of the Mekong: a single sighting (of a male and an unsexed individual on 3 April 2010) at Kok Kawdinpiang (in Phou Gnouey Production Forest Area, Vientiane province, at about 18°18.1’N 101°46.8’E) (SUFORD in press). The presence of grantia on the south bank of the Mekong, and of viridis on the north, evidently indicates that this major river does not act as a complete boundary separating these two taxa, and therefore that northern Laos and northern Thailand should encompass a zone of contact between them. In spite of this, however, there are no reports that directly indicate their coexistence at any site. Relatively little is known of the biology of either species. The vocalisations of the two are extremely similar. These include a dry undulating cackle, somewhat reminiscent of one of the calls of Bay Woodpecker Blythipicus pyrrhotis (‘rattle call’ in Short 1973), and piercing even-toned kweep notes. Both species drum (Winkler & Christie 2002) and the pattern of drumming described for G. grantia (‘…initially very rapid and clearly and gradually decelerates…start rate 30, end rate 15 taps/s’, of roughly 1.5 s duration: Rasmussen & Anderton 2005) is similar to that of G. viridis (PDR recording from southern Thailand, deposited with Avian Vocalizations Center, Michigan State University). |
|
| Progress
of observations Intermittent sightings of Gecinulus woodpeckers were made by JMH on and near his farm at Ban Saen Jai from August 2009 onwards. On 10 March 2010, at 09h00, a Gecinulus woodpecker was revealed as the source of a loud, insistent tapping, suggesting the excavation of a cavity, near the vicinity of a small pond at the bottom of a steep wooded gully. The nest cavity itself was discovered by JMH a little after 09h30 that day, when he flushed a woodpecker at close range from a dense clump of large-culm bamboos. The female woodpecker was again seen in the vicinity at c.11h00 while, at 15h00, a male woodpecker, heard tapping from within the nest cavity, was seen when its head protruded from the nest-entrance, revealing red on the crown. PDR joined JMH at the site during 13–15 March, when both male and female were seen with heads protruding from the nest cavity on different occasions. Bouts of drumming were heard and there were long periods when tapping could also be heard, apparently emanating from within the nest-cavity. Observations were kept to a minimum so as to avoid disturbing the nesting pair, then assumed to be either in the process of laying, or already in the early stages of incubation. On 15 March, JMH watched the female enter the nest at 17h30. JMH continued observations intermittently, observing the head of the female protruding from the nest cavity on two occasions during 23–25 March. During observations of the nest from a blind, 20 m distant, on 3 April, 14h30–17h30, RK secured photographs of both breeding adults and observed both sexes removing faecal sacs from the nest, indicating that the young had hatched. JMH watched the nest further during 4–11 April and 14–18 April, and was joined by both PDR and JSS during 14–17 April. The young could be heard calling from within the nest from at least 8 April onwards, and both adults were highly vocal in the vicinity of the nest, giving chattering and kweep notes. Drumming was intermittently heard. Recordings of the calls of adults, made in March, and of chicks, during April, were deposited with the Avian Vocalizations Center, Michigan State University. On 16 April the female was caught in a 12 m superfine mistnet erected in front of the nest cavity as she was exiting the nest, c. 07h30, after having fed the nestlings. She was examined, photographed, measured and ringed. Two feathers were retained for possible future DNA assay. Only one nestling, the presumed female (see below), remained in the nest on 17 April (its presumed male sibling was heard calling nearby but could not be located). The female chick was also thought to have left the nest later that day, and by the morning of 18 April neither adults nor young could be detected anywhere in the vicinity. The section of bamboo containing the empty nest was removed later that day in order to examine the nest contents and dimensions. JMH observed presumably the same pair of woodpeckers (the female was ringed) at a recently excavated cavity in an adjacent stem in the same bamboo clump, during 11–16 June 2010. On two occasions in the early morning the female was seen with head protruding from the nest cavity, and on the first of these, when the female exited, the male promptly entered, suggesting a possible repeat nesting attempt. However, no further observations were made after 16 June and no firm conclusions could be drawn. Winkler & Christie (2002) specifically remark that daytime frequenting of roost-holes by woodpeckers may, particularly in the tropics, be misinterpreted as breeding behaviour. The birds were again searched for, but not found, by JMH in the following year (2011). |
|
| Nest
Site The nest site was situated at the north-eastern edge of a clump of mainly dead or senescent flowering bamboos on the steep flank of a deep gully that supported a narrow (c.60 m wide) band of dense remnant, secondary, semi-evergreen vegetation. The canopy cover was estimated at 70%. In the gully bottom a seasonal stream flowed into a small, dammed pond, which holds water yearround. During the period of observation, the height of the dry season, the stream had dried up, although its bed remained moist. At the pond the gully joined another wooded gully, forming part of a continuous ribbon of dense vegetation that drained to the north. A path along the northern side of the gully, half-way upslope, passed within 6 m of the nest cavity, which was slightly above head height. The steep bank immediately above the path was densely covered with small trees, bushes and herbage, providing a vantage point where a blind was constructed at a distance of 20 m from the nest, and looking down on to it, so as to observe the birds without disturbance. The path was seldom used except by occasional hunters, bamboo cutters and wandering cattle herders. Almost daily in the late afternoon herders brought their cattle to the pond below the nest for water. There was no evidence that this significantly disturbed the birds. |
|
| Nest
Description The entrance hole was towards the upper margin of the 11th internode section of the stem of dead bamboo, Gigantochloa apus (Schulz) Kurtz. (Gramineae, Bambusoideae), c.4 m above the ground. The bamboo stem, which contained the nest hole, had been cut at the base earlier in the year and left, dead, in situ, by bamboo cutters, and was angled at roughly 60°. The top of the hole was 8.0 cm from the lower edge of the upper node and its base was 49.8 cm above the top edge of the lower node. The external circumference of the bamboo stem measured at the centre of the hole was 34.8 cm and the internal diameter of the nest cavity c.9 cm. The entrance hole was hexagonal in shape with the vertical axis longer than the horizontal axis (the apex of the hexagon to its lowest point was 6.8 cm and the parallel sides of the nest-entrance were 3.9 cm apart). The lower rim of the nest-entrance was highly abraded (Figure 2). The internal height from the bottom of the nest cavity to the lowest point of the cavity entrance (the distance the young would have to climb to be fed at the nest entrance) was 47 cm. The interior wall of the bamboo was smooth above the nest hole, but vertically scored and shredded from the level of the hole to the floor of the cavity, and some of this shredded bamboo fibre apparently contributed to a 6 cm deep layer of black, soft, fine, fibrous vegetable matter, infested with small (c.1 cm), thin, white maggot-like insect larvae, on the cavity floor. The contents of the nest were preserved in alcohol for further analysis. The site of the second cavity, found in June, was in a similar bamboo stem, which was dead (after flowering) but had not been cut. |
|
| The
Nestlings The heads of the two nestlings were seen protruding from the nesthole on 15 April when a presumed male nestling could be seen to have a red mid- and hind-crown, lacking in a presumed female nestling. |
|
| Appearance
of the breeding pair The male bird appeared like a more or less typical G. viridis with greenish body plumage. However, the crown was not solidly red and did not extend fully onto the nape. The tail appeared unmarked when seen from above, but the primaries and secondaries had indistinct pale bars, with a slight rufous tinge evident at times (Figure 2). |
|
 Figure 2. Male Gecinulus at nest, Ban Saen Jai, 3 April 2010. (Rungsrit Kanjanavanit) |
 Figure 4. Lateral view female Gecinulus in the hand, Ban Saen Jai, 16 April 2010. Note the extensively barred primaries and secondaries, and rufous-tinged secondaries. (P. D. Round) |
 Figure 3. Female Gecinulus at nest, Ban Saen Jai, 3 April 2010. (Rungsrit Kanjanavanit) |
 Figure 5. Rump, uppertail-coverts and spread tail of female Gecinulus in the hand, Ban Saen Jai, 16 April 2010. Note the prominent barring on inner and outer webs of rectrices 1–5. (P. D. Round) |
| The female differed markedly from typical G. viridis females in showing rufous-tinged secondaries, and prominent broad whitish barring on the primaries, secondaries and all rectrices (Figure 3). The conspicuous broad, sharply contrasted pale barring on remiges and rectrices was easily visible in the field, both at rest and in flight. | |
| Description
of female in the hand (Figures 4, 5) Throat and forecrown unmarked, pale brownish. Mid-crown, hindcrown and ear-coverts yellowish-olive. Mantle and lower back bronze-olive (olive-green); upperwing-coverts concolorous dull bronze-green. Rump feathers extensively tipped (maroon) reddish and uppertail-coverts dull bronze-olive. Underparts (breast, belly and undertail-coverts) dull, dark olive. Prominent white spotting/transverse barring on both outer and inner webs of all primaries (brighter on inner webs). Bright white spotting/transverse barring on all secondaries (less distinct on outer webs). Outer webs of all secondaries rufous-tinged, forming a slightly rufous panel on the closed wing. Rectrices dark olive-brown, with rufescent-olive outer webs. Rectrices 1–4 with four clear white bars, visible on both webs; rectrix 5 with three white bars, visible on the inner web only. Rectrices 1–5 were modified with pointed tips and stiff shafts. Rectrix 6 was short, unstiffened and unmarked, less than half the length of the central pair, as is more or less typical for woodpeckers. Iris ruby-red; narrow grey orbital ring; bill bluish-white, legs and feet olive-green. Wing length 131 mm (maximum chord), tail 91 mm, bill (to skull) 28.2 mm, tarsus 26.9 mm, weight 72.9 g. Secondary 6 right wing was old, unmoulted, as were secondaries 7 and 8 on the left wing. |
|
| Comparison
with specimens Detailed comparison of photographs of the Ban Saen Jai nesting pair was made by PDR with four male (or male-plumaged) specimens and one female specimen of G. v. viridis in CTNRC (Figure 6). The photographs were also compared with five male and two female Thai and Tenasserim G. v. viridis specimens; a further 18 female G. v. viridis specimens from elsewhere in the range; and with specimens of G. v. robinsoni from the Thai-Malay Peninsula, and three taxa of G. grantia (excluding ‘poilanei’). The latter comparisons were made by JSS at BMNH, and by PDR and JSS together from photographs. |
|
 Figure 6. Dorsal view of four Thai-taken male/male-plumaged specimens, and one female specimen, of Gecinulus viridis. Note the restricted areas of red on the hind-crown on the right-hand-most red-crowned individual, CTNRC 53-3344, from Mae Jan, Chiang Rai. (P. D. Round/Centre for Thai National Reference Collections) |
|
| Neither
males nor females of any G. viridis specimens examined
showed any rufous cast on the secondaries or elsewhere, nor any
clearly visible tail barring when the tail was examined from above.
Tail barring was restricted to small white spots on the inner webs
of rectrices 2–5, with the central pair of rectrices either
unmarked or with one or two small white spots on the basal portion
of the inner web. A pattern of vague barring on the primaries and
secondaries in G. v. viridis specimens was never as contrasted
as in the Ban Saen Jai bird, and mainly restricted to white spots
on the inner webs. Faint barring, usually visible on the outer webs
in females, was never as prominent as on the Ban Saen Jai bird. Prominent wing and tail barring is characteristic of G. grantia. However, the pale bars are strongly rufous rather than whitish in that species, and are broader, more than half as broad as the intervening dark brown bars. In addition, the mantle and wings in G. grantia are strongly chestnut-red, the sexes scarcely differing in hue. While G. g. indochinensis is slightly less intensely reddish than the nominate race it nevertheless remains strongly chestnut-rufous (Figure 7). The southern Chinese G. g. viridanus is dark rufous, less strongly chestnut on the upperparts, which have some greenish feathers mixed in, but it retains prominent wing and tail barring in which the pale bars are rufous (Figure 8). |
|
 Figure 7. Dorsal view of three specimens of G. grantia indochinensis from Lo-Tiao, Bokeo, Laos. From right to left MCZ 267140 (male); MCZ 267141 (female), MCZ 267142 (male). (Jeremiah Trimble, Museum of Comparative Zoology, Harvard University /© President and Fellows of Harvard College) |
 Figure 8. Dorsal view of two specimens of G. grantia viridanus, BMNH 1900.1.18.328 (male, left) and BMNH 1905.12.24.423 (female, right). (J. Steward/ © Natural History Museum) |
| No
specimens of any taxon of either G. viridis or G. grantia
examined in collections precisely resembled the Ban Saen Jai female.
The latter appeared more or less intermediate between the two: in
overall plumage tones more akin to viridis than grantia, yet with
a pronounced rufous cast on the secondaries, and clear, broad whitish,
well-contrasted bars on primaries, secondaries and tail feathers
that were not shown by any other viridis specimen. Photographs of King’s female specimen from Chiang Khong (USNM 534656) also revealed that it is somewhat intermediate in appearance. It differs from any other G. g. indochinensis or G. g. viridanus specimen in being markedly and evenly green-tinged on the mantle, recalling the Saen Jai bird, although it possesses rufoustinged, rather than whitish, bars on the folded wing. The tail-bars, however, are whitish rather than rufous-tinged and neither as broad nor as boldly contrasted as in any G. grantia (Figure 9). |
|
 Figure 9. USNM 534656 (dorsal view and lateral view), collected Chiang Khong, Chiang Rai, northern Thailand, 26 April 1964, by B. King. Note the extensively greenish mantle which is atypical for any subspecies of Gecinulus grantia. (J. Dean/© National Museum of Natural History, Smithsonian Institution) |
|
| Gecinulus viridis and G. grantia also differ in the patterning of red on the crown of males. In G. viridis the mid-crown and hindcrown and nape are solidly red. In G. grantia the red on the crown is less extensive, pinkish-red, broken on the hind-crown and does not extend to the nape (Figures 7, 8). In this respect, the Ban Saen Jai male was unusual among G. viridis in that the red on the hindcrown was less extensive than is typical for the species. Of four maleplumaged specimens in the CTNRC collection, three (two from Kanchanaburi, south-west Thailand, and one, market-purchased, provenance unknown) have extensive and solidly red crowns. A fourth (specimen no. 53-3344; second from right, Figure 6) lacks solid red on the hind-crown. This specimen, labelled as a female, probably in error (the label reported the gonads as small), was collected at Huai Mae Salaep, Mae Jan district, Chiang Rai, (c.20°11’N 99°42’E), only a few kilometres from Ban Saen Jai. In terms of its weak wing and tail-feather barring and olive-green body coloration, the specimen looked typical for G. viridis. | |
| DISCUSSION The existence of a female Gecinulus, clearly outside the normal range of variation of Bamboo Woodpecker, somewhat intermediate in plumage between G. viridis and G. grantia, and the existence of another female Gecinulus (USNM 534656, labelled G. g. indochinensis) from the same general area (Chiang Rai province) which differs markedly from topotypical G. g. indochinenis from further north and east in Indochina, suggests that viridis and grantia may intergrade in this region of northern Thailand and possibly adjacent northern Laos. The coincidence of reduced red on the crown in two Chiang Rai male G.viridis, the Ban Saen Jai nesting bird and CTNRC 53-3344 from nearby Huai Mae Salaep, may possibly also be significant. Is reduced red on the crowns of males typical for Chiang Rai/northern Thailand G. viridis? Might this, in fact, be further evidence of intergradation between G. viridis and G. grantia? The only other G. viridis specimen from the Thai– Lao border region (the Ban Moung Liap bird, BMNH 1955:1.2505) seems also to possess a less solidly red hind-crown, although in other respects it appears typical for G. viridis. Although both species occur in northern Laos there appear to be no reports of them occurring in close proximity at the same location (Figure 1). The few specimens of Pale-headed Woodpecker in Laos closest to the areas supporting Bamboo Woodpecker for which photographs were examined are typical chestnut-backed G. grantia indochinensis, with strongly and broadly barred wings and tails, lacking any intermediate characters. The most significant, since they were collected only an estimated 20 km north of the site of King’s presumed hybrid (albeit on the opposite bank of the Mekong), were the three MCZ specimens from Bokeo province, at Lo-Tiao (Figure 7). Since Gecinulus woodpeckers are relatively shy and hard to approach and observe, the similarity of the vocalisations of the two species may mean that fleeting sight records collected during faunal surveys within the zone of contact or sympatry may not be assignable as to species with 100% confidence. (So far, purely aural records are not known to have provided the basis for any northern Lao reports of either species: J. W. Duckworth in litt.). If G. grantia and G. viridis do intergrade widely, then intermediates might be expected to show a highly variable mix of characters, and those individuals with only subtle differences from either parent species might easily be overlooked. On the other hand, if both occur sympatrically without intergradation in their zone of contact, such sympatry might remain undetected if one species was rare, and the other relatively common at any given site. Information on the extent of ecological differences between these two taxa is scant. Since both are associated with large-culm bamboos, most if not all nests may be expected to be situated in cavities in bamboos. The only nest described for G. viridis, from the Thai-Malay Peninsula, was excavated in the bamboo Gigantochloa scortechinii Gamb. (Wells 1999), while both Short (1973) and PDR have seen holes presumed to have been excavated by G. viridis in large-culm bamboos at Thai localities where the species is present. There appear to be no nest records of G. grantia anywhere in its Indochinese range, and the only nests described for G. grantia by Baker (1927), from the northern Indian subcontinent, were apparently in tree-stumps. Too few nests of either species have been found to know whether reported differences are typical, or whether nest-sites in either or both species may be situated in either tree stumps or bamboos, depending on availability. However, Baker’s descriptions of nests of G. grantia may be questionable, since his written work contains inconsistencies and discrepancies from that of other workers, and many of his findings have been either discounted or questioned. Until convincing evidence is presented that refutes this, it should be assumed that G. grantia and G. viridis are very similar in their ecology. Efforts are needed in northern Thailand and northern Laos to discover how frequent intermediate-plumaged Gecinulus woodpeckers are, and investigate the ecological and taxonomic relations of G. grantia and G. viridis. Chiang Rai province, north and east of the area of the present sighting, is an obvious priority area for survey, as are sites in Laos where the ranges of grantia and viridis approach closely: Bokeo province; Vientiane province and municipality; and northern Laos west of the Mekong (Xaignabouli province). Arguably, however, almost all of northern Laos, where relatively few surveys have been implemented, and in which the status of Gecinulus woodpeckers remains largely unknown, would repay survey. Gecinulus grantia and G. viridis presumably diverged from a common ancestor during a previous period of forest fragmentation. The presence of apparent plumage intergrades suggests that these taxa have since come into renewed contact before isolating mechanisms between them have been fully developed. A review of hybrid zones in birds is provided by Price (2008) and, indeed, hybrid zones may prove to be relatively frequent among parapatric taxa in the tropics. Manakins Manacus offer well-studied examples from the Neotropics (Brumfield et al. 2001, Stein & Uy 2006), while hybridisation is also documented among some Melanerpes woodpecker species (Short 1982) and in North American flickers Colaptes (Short 1965, Moore & Price 1993). Given their marked divergence in plumage patterns, Pale-headed Woodpecker and Bamboo Woodpecker qualify as species using Helbig et al.’s (2002) criterion for assigning taxonomic rank (hybridisation is rare, making it unlikely that their gene pools will ever merge). But if intermediate-plumaged birds, apparently caused by interbreeding, prove to be frequent within the zone of contact, a re-examination of their taxonomic status might be necessitated. Even so, provided that the postulated hybrid zone is narrow in relation to the total ranges of the taxa, indicating barriers to gene flow, the two would probably still continue to be treated as species or (following Helbig et al. 2002) semi-species. A simple scoring system based on phenotypic characters, applied to all Gecinulus sight and photographic records, trapped birds and museum specimens from within the likely contact zone, might help to elucidate the extent and pattern of introgression between the two. |
|
| ACKNOWLEDGEMENTS James Dean in USNM, Mary Hennen at FMNH, and Jeremiah Trimble at MCZ kindly photographed specimens of G. grantia on our behalf. BMNH and CTNRC kindly allowed access to their holdings, and we thank Robert Prys-Jones at the former institution, and Surachit Waengsothon at the latter, and their respective staffs, for their kind assistance, especially Hein van Grouw at BMNH for providing label data and locating and photographing an additional Gecinulus specimen on our behalf. The Harrison Institute, Kent, UK (supported by the Darwin Initiative), and the Wildlife Conservation Society, New York, provided copies of some key references and we are particularly grateful to Paul Bates and Kerry Prendergast, respectively. Warren Brockelman, James Dean, Edward Dickinson, Will Duckworth, Ben King, Joe Tobias and David Wells commented on drafts of this manuscript. Will Duckworth, in particular, drew our attention to some key Lao references and made innumerable suggestions that improved this paper. We also thank two anonymous referees. Jerome Fuchs and Eric Pasquet provided further information on Lao Gecinulus records, as did Le Trong Trai, Simon Mahood, John Pilgrim and Jack Tordoff for those from Vietnam. J. F. Maxwell, Chiang Mai University Herbarium, identified the species of bamboo containing our woodpecker nest. Perawit Insuan kindly prepared the map. Philip Round is supported by The Wetland Trust. |
|
REFERENCES Bangs, O. & Van Tyne, J. (1931) Birds of the Kelley–Roosevelts expedition to French Indochina. Publ. Field Mus. Nat. Hist. (Zool. Ser.) 18: 33–119. Brumfield, R. T., Jernigan, R. W., McDonald, D. B. & Braun, M. J. (2001) Evolutionary implications of divergent clines in an avian (Manacus: Aves) hybrid zone. Evolution 55: 2070–2087. Deignan, H. G. (1950) Five new races of birds from south-eastern Asia. Zoologica 35 (2): 127–128. Delacour, J. (1951) Commentaires, modifications et additions à la liste des oiseaux d’Indochine Français. Oiseau 21: 1–32. Delacour, J.
& Greenway, J. C. (1940) Liste des oiseaux recueillis dans la
province du Haut-Mekong et le royaume de Luang-Prabang. Oiseau
et Dickinson, E. C. ed. (2003) The Howard & Moore complete checklist of birds of the world. Third edition. Princeton: Princeton University Press. Duckworth,
J. W. (1996) Bird and mammal records from the Sangthong District,
Vientiane Municipality, Laos, in 1996. Nat. Hist. Bull. Siam
Soc. Duckworth, J. W., Salter, R. E. & Khounboline, K. compilers (1999) Wildlife in Lao PDR: 1999 status report. Vientiane: IUCN-The World Conservation Union/ Wildlife Conservation Society/Centre for Protected Areas and Watershed Management. Evans, T. D. (2001) Ornithological records from Savannakhet Province, Lao PDR. Forktail 17: 21–28.# Fuchs, J., Cibois, A., Duckworth, J. W., Eve, R., Robichaud, W. G., Tizard, T. & van Gansberghe, D. (2007) Birds of Phongsaly province and the Nam Ou river, Laos. Forktail 23: 22–86. Helbig, A. J., Knox, A. G., Parkin, D. T., Sangster, G., & Collinson, M. (2002) Guidelines for assigning species rank. Ibis 144: 518–525. King. B. (2007) Some 1960s additions to the list of Thailand’s birds. Nat. Hist. Bull. Siam Soc. 55 (1): 105–119. King, B., Dickinson, E. C. & Woodcock, M. W. (1975) Field guide to the birds of South-East Asia. London: Collins. Lekagul, B. & Round, P. D. (1991) A guide to the birds of Thailand. Bangkok: Saha Karn Bhaet. Moore, W. S.
& Price, J. T. (1993) Nature of selection in the northern flicker
hybrid zone and its implications for speciation theory. Pp.196–225
in Pasquet, E. (1997) Oiseaux, Pp.1–4 in Rapport Muséum NHNP–Forespace Avril 1997. Paris : Muséum National d’Histoire Naturelle. Price, T. D. (2008) Speciation in birds. Greenwood Village, USA: Roberts & Co. Rasmussen, P. C. & Anderton, J. C. (2005) Birds of South Asia: the Ripley guide. Washington, D. C. and Barcelona: Smithsonian Institution and Lynx Edicions. Robinson, H. C. & Kloss, C. B. (1931) Some birds from Siam and Laos (Middle Mekong). Ibis 13(1): 319–341. Robson, C. (2008) A field guide to the birds of South-East Asia. London: New Holland. Short, L. L. (1965) Hybridization in the flickers (Colaptes) of North America. Bull. Amer. Mus. Nat. Hist. 129: 307–428. Short, L. L. (1973) Habits of some Asian woodpeckers (Aves: Picidae). Bull. Amer. Mus. Nat. Hist. 152: 332–336. Short. L. L. (1982) Woodpeckers of the world. Cinnaminson, New Jersey and Dordrecht, Holland: Foris Publications. Stein, A. C.
& Uy, J. A. C. (2006) Unidirectional introgression of a sexually
selected trait across an avian hybrid zone: a role for female choice? SUFORD (Sustainable Forestry for Rural Development) project (in press) Preliminary biodiversity assessment and management recommendations of SUFORD-AF Production Forest Areas. Vientiane: SUFORD project, Department of Forestry. Thewlis, R.M., Timmins, R. J., Evans, T. D. & Duckworth, J. W. (1998) The conservation status of birds in Lao PDR. Bird Conserv. Internatn. 8 (suppl.): 1–159. Wells, D. R. (1999) The birds of the Thai-Malay Peninsula, 1. Non-passerines. London: Academic Press. Winkler, H. & Christie, D. A. (2002) Family Picidae (woodpeckers). Pp.296– 555 in J. del Hoyo, A. Elliott, & J. Sargatal, eds. Handbook of the birds of the world, 7. Barcelona: Lynx Edicions. |
|
| Kindly
submitted by: Philip D. ROUND, Regional Representative, The Wetland Trust, and Assistant Professor, Department of Biology, Faculty of Science, Mahidol University, Rama 6 Road, Bangkok 10400, Thailand. Email: philip.rou@mahidol.ac.th John M. HOBDAY, 210/3 Mu 1, Soi Kiengdoi, T. Chang Pheuak, A. Muang, Chiang Mai 50300, Thailand. Rungsrit KANJANAVANIT, Lanna Bird and Nature Conservation Club, 76/1 Mu 14, Soi 5 Suthep Road, A. Muang, Chiang Mai 50200, Thailand. James S. STEWARD, Department of Biology, Faculty of Science, Mahidol University, Rama 6 Road, Bangkok 10400, Thailand. |
|
| Tweet | |

A Guide to Birdwatching in Thailand. Copyright © 2004-2017 thaibirding.com. All rights reserved.