
|
||||||||||
|
||||||||||
| Favorite Tweets by @thaibirding | ||||||||||
|
||||||||||
 Buy Birds of Thailand on Amazon.co.uk |
||||||||||
| Site Map ; Contributors |
| A
comparison of bird communities in mixed fruit orchards and natural
forest at Khao Luang, Southern Thailand. By Philip D. Round, George A. Gale & Warren Y. Brockelman |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tweet | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Note: This article was originally published in Biodiversity and Conservation 15 (2006) and was kindly submitted by Phil Round. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ABSTRACT The avifauna of a mixed fruit orchard and nearby isolated forest patch on the mountain of Khao Luang, southern Thailand, was compared with that in natural forest. The orchard was about 75% as rich in bird species as the forest and was dominated by smaller frugivores, nectarivores and widespread generalists. Sundaic birds contributed 26% of sightings in the orchard, and understorey insectivores were poorly represented. The avifauna of the 4.5-ha forest patch was similarly depauperate and bore greater resemblance to that in the orchard than to that in forest. These results have implications for management since increasing emphasis is being placed upon the rights of local communities to manage and exploit resources in protected areas. While agricultural diversification may assist in restoring modest levels of diversity in areas already degraded or committed to human use, it should not be seen as a substitute for conventional protection of forest and wildlife through exclusion of such use. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| INTRODUCTION In recent years increased emphasis has been placed on the rights of local communities to both manage and utilise the resources of forests, including those inside protected areas. Social scientists have argued, primarily from the point of view of social justice, that the forests should be ‘returned to the people’ (e.g. Ramitanondh 1989; Ghimire and Pimbert 1997). Some resource managers and community foresters have argued that parks and sanctuaries have failed to conserve biodiversity as intended, and that conservation would be better served if local people were involved in harvesting, and managing, their resources (Fisher 1995; Ghimire and Pimbert 1997; Makarabhirom 1998; Scott 1998; ICEM 2003). This view is widely held even though Bruner et al. (2001) during a wide survey of tropical protected areas, concluded that conventional protection, usually through exclusion or strict regulation of human use, was highly effective in protecting biodiversity through reducing land-clearance. It is important that baseline studies are conducted to assess the actual and potential human impacts of human use, and modification of vegetation, upon biodiversity before any changes in protected area policy are made. Although claims are often made of the historic role of communities in protecting forest and biodiversity, the capacity of communities to self-govern their natural resources cannot be assumed. In one of the few scientifically based comparisons conducted, Salafsky et al. (2001) reviewed 39 project sites in Asia and the Pacific, and found that community-based management strategies only worked under a limited set of circumstances, in which a mix of strategies including direct protection, management and restoration, policy and advocacy as well as education and awareness was used. There is a surprising scarcity of studies that have quantified the biodiversity values of existing community forests or intensively man-managed forests compared with undisturbed forest. Complex agroforestry systems with fruit trees are found in or near forest areas in many parts of South and Southeast Asia (Michon and de Foresta 1990; Gujral 1991; Wiersum 1991). These are multi-storyed associations of planted or selected native species of trees, shrubs, and herbs that supply products both for household use and for local markets. The best-known example in Thailand is at Ban Khiriwong, Lan Saka District, Nakhon Si Thammarat Province, on the steep slopes of the mountain of Khao Luang, where forest orchards or ‘suan somrom’ have operated for over 100 years (Makarabhirom 1991; Juiprik 1997). The Khiriwong orchards are often cited as a good example of peoples’ coexistence with forest by the villagers who enthusiastically promote themselves as forest managers (Tourism Authority of Thailand 2000). The aim of this survey was to compare some ecological attributes of the Khiriwong mixed orchards with those of ‘natural forest’ nearby, in Khao Luang National Park. Birds were selected for study here because they are diverse, with (in most cases) well-known habitat requirements and general bionomics (e.g. Round 1988; Lekagul and Round 1991), and they are relatively easily surveyed and detected, especially given familiarity with their vocalisations. They are therefore especially useful as indicators of biodiversity. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| STUDY
AREAS Three sites were selected for study: orchards and a forest patch near Khiriwong village; and a representative example of forest at Krung Ching, some 34 km distant on the same mountain. The latter site was the closest available site that was topographically similar to Khiriwong, being situated along a stream valley, yet which still had relatively intact lowland forest. Khiriwong orchard The study area lay on foothills and lower submontane slopes (160–320 m elevation) in a stream valley on the south-eastern flank of Khao Luang at ca.8o 26.5' N; 99o 45.5' E (Figure 1). Vegetation was predominantly orchard, formed mostly by Durio zibethinus Murray, with 34%of the total canopy volume, with Parkia speciosa Hassk. ranked next with about 16%. Other canopy trees, included Sandoricum koetjape (Burm. f) Merr., and Areca catechu L., planted for their fruits, together with Michelia champaca L., planted for timber. Trees comprising the middle storey included Garcinia mangostana L. (13% of canopy volume), Lansium domesticum Correa, and Bouea macrophylla Griff. Relatively little of the canopy volume (22%) lay above 20 m. The understorey was sparse, formed by a few bananas, Musa acuminata Colla, and the ground layer was predominantly of grasses, vines and herbs, 1 m high, with some tapioca. A few native trees remained, especially in ravines and along the banks of the stream. The total orchard area covered by the bird census was approximately 50 ha. Forest patch One 4.5-ha fragment, surrounded by and near the upper limit of the fruit orchards, at 510–600 m elevation (8o 26.3' N; 99o 44.5' E), was selected for survey. The fragment contained a mix of disturbed and selectively logged forest and while the species composition was not surveyed in detail, the species diversity was clearly higher, it had about five times as many woody stems as the orchard. The forest patch also had approximately twice the height and foliage mass of the orchard, with 76% of the canopy cover lying above 20 m. There was also a dense lower understorey of woody plants in contrast to the orchard. The forest patch was completely encircled by orchards and banana plantations and a small brook ran through its centre. Krung Ching forest The third study area was an area of mature forest at Krung Ching, Nop Phitam District, inside the national park, on the northern flank of Khao Luang, along the catchment of the Krung Ching stream, at roughly 8o 43' N; 99p 41' E (Figure 1). Although the vegetation was not surveyed, the area was dominated by continuous, tall, mixed dipterocarp forest that was either unlogged, or had only been lightly logged in the past. The vegetation was tall and multi-storeyed, and rich in palms and lianas. The area was relatively well-watered, with a number of small damp patches and forest streams. It covered a range of elevations similar to the Khiriwong orchards, 180–340 m, and was therefore appropriate for comparison, though differed somewhat in its less steep topography, encompassing a plateau of ca. 15 km2 at roughly 300 m elevation. Data collection was limited to the northern and eastern margins of the plateau, against the foothills, so as to cover an area of c. 50 ha as similar in topography as possible to Khiriwong. These three study sites are henceforth referred to as Khiriwong orchard, Khiriwong forest fragment (or forest patch), and Krung Ching forest. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
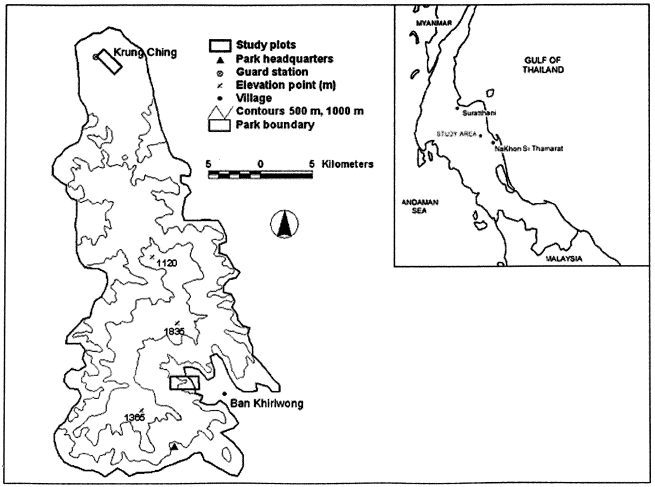 Figure 1 : Locations of study areas in Khao Luang National Park. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| METHODS Khiriwong orchard was visited during 28–30 October 2000; the Khiriwong forest patch during 20–23 November 2001 and Krung Ching during 7–10 April 2001. At each site the observer walked slowly through each area, recording all birds seen or heard on ‘MacKinnnon Lists’ (MacKinnon and Phillipps 1993; Bibby et al. 1998). In this method, all species are listed, up to a predetermined number of species, when a new list is started. Each species only appears once per list, regardless of whether the encounter is with a single individual or with a flock of several individuals. Although MacKinnon Lists are less useful in terms of quantifying the evenness of the community structure compared to traditional point counts, they have been shown to perform well in terms estimating species richness (O’Dea et al. 2004). We attempted to obtain 10 lists of 20 species in each habitat because experience elsewhere in Thailand and SE Asia has shown this to be manageable and efficient (Round 1998, 1999). In the Khiriwong orchard, data collection was made mostly along transects spaced 100 m apart, also used to collect vegetation data, although was not limited to the transects alone, The Khiriwong forest patch, however, was only large enough to obtain nine independent lists. In the Krung Ching site 11 lists were obtained. Both the Khiriwong orchard survey and the Krung Ching survey were carried out by the same observer while the Khiriwong hill-slope patch survey was implemented by a different observer. The observers had a high familiarity with calls and songs of most species. Birds identified by sound were only counted if estimated to be within 30 m, in order to avoid over-counting species with loud, obtrusive calls such as barbets. Both migrant and resident species were tallied, since surveys of the three sites took place during the Palearctic winter, when a reasonably complete complement of migrant (non-breeding) species could be expected to be present. Efforts were made to spread coverage evenly throughout the areas, in order to avoid bias from repeated observations of the same individual birds. Randomized species accumulation curves were drawn, and diversity indices calculated, for each site using EstimateS (Colwell 2004). The percentage of observations contributed by each species was also calculated as a means of objectively estimating relative abundance. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| RESULTS A total of 123 species of birds was recorded at the three sites combined (Appendix 1). Fifty-nine species were recorded on 10 lists (average 56.6 species on nine lists) in the Khiriwong orchard; 51 species on nine lists in the Khiriwong forest patch, and 82 species on 11 lists (average 75.8 species on nine lists) at Krung Ching (Table 1, Figure 2). Fifteen species were shared among all three sites; 18 species were found in the Khiriwong orchard but not at the other two sites, 10 species were found only in the Khiriwong forest patch, while 41 species, exactly half of all those found, were unique to Krung Ching. The two forest sites had 13 species in common that were not found in the orchard, while the forest patch and the orchard shared 13 species that were not found in Krung Ching (Appendix 1). The three sites therefore had rather different bird communities, the two Khiriwong sites having slightly less than half their species in common (Sorensen qualitative index, CS = 0.483: Table 2). The most distinct site was the lowland forest at Krung Ching (CS = 0. 383 compared with the orchard, and 0.403 with the Khiriwong forest patch: Table 2). The species accumulation curves for all sites were relatively steep, in no case reaching an asymptote, although the forest patch curve appeared to be flattening (Figure 2). However, the list for Krung Ching climbed more steeply than at either of the Khiriwong sites, reflecting the much greater species richness of the forest. Chao 2 estimates of species richness were 86.7 for the Khiriwong orchards, and 53.3 for the forest patch compared with 115.1 for Krung Ching forest (Table 1). A Fisher’s lndex (a) value of 47.4 was obtained for the forest site, compared with 28.2 for the orchard and 23.7 for the forest patch (Table 1). The frequency distribution data from Krung Ching and the Khiriwong Forest Patch both fitted the log series (v2 = 4.66 and v2 = 4.68, respectively; for 3 degrees of freedom, p>0.10), while that for Khiriwong orchards was significantly different (v2 = 11. 36; for 3 degrees of freedom, p<0.01). However, Leigh and de Lao (2000) make persuasive arguments for using Fisher’s Index for diversity comparisons, even when the data does not necessarily fit a log-series. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Table
1 : A comparison of bird diversity among three study sites.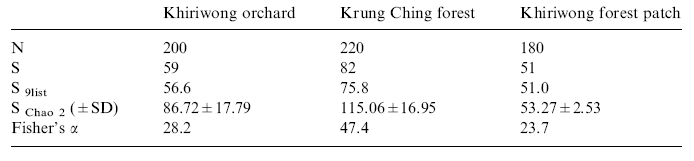 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
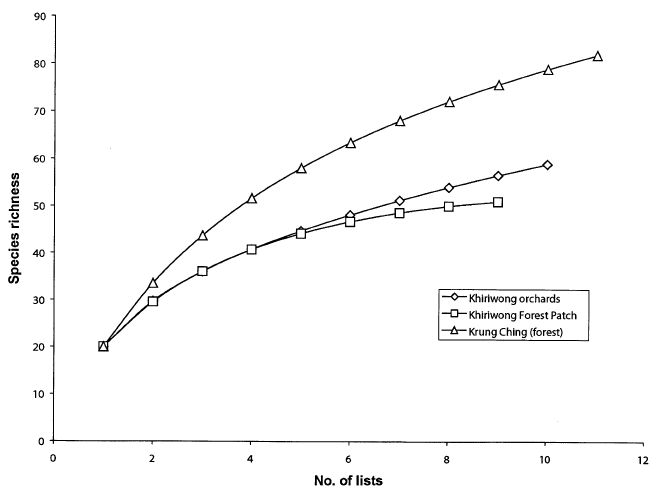 Figure 2 : Species accumulation curves for orchard, forest patch and forest, Khao Luang National Park. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Table
2 : Coefficients of similarity (Cs) among the three sites. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Further comments are made with respect to each site below. Species nomenclature follows Round (2000). Khiriwong orchard The bird community at Khiriwong was heavily biased towards edge-inhabiting species, and generalist species with a wide distributional range throughout Thailand. The most abundant species in the orchards were the omnivorous bulbuls, Pycnonotus finlaysoni and Iole olivacea (each recorded on 100% of lists). Nectar-feeders, such as Arachnothera longirostra and Anthreptes malacensis, the nectarivorous/frugivorous Loriculus vernalis, and the small frugivore Dicaeum trigonostigma were also abundant, occurring on more than two-thirds of lists. The migrant arboreal sallying insectivore, Muscicapa dauurica, and the foliage-gleaning Gerygone sulphurea were frequent in the canopy, and another foliage gleaner, Prinia rufescens, in the understory and ground storey. The only frequent terrestrial insectivore was Pellorneum ruficeps. Of the total of 59 species, 18 species (and three of the 20 most abundant species, Iole olivacea, Dicaeum trigonostigma and Arachnothera chrysogenys) were of Sundaic affinity, and together accounted for 52 of 200 bird registrations (26%). The remainder were species that were widespread in the Indochinese subregion. Four species of babblers together accounted for 8% of all bird registrations, and none of these species (Macronous gularis, Pellorneum ruficeps, Stachyris nigriceps and Yuhina zantholeuca) was strictly Sundaic, all being more or less common and widely distributed throughout the country. Only one species recorded at Khiriwong, Aviceda jerdoni, was considered as nationally near-threatened (Round 2000). Khiriwong forest patch The most abundant species, occurring on 100% of lists, were Alophoixus ochraceus (an omnivore), Arachnothera longirostra, the frugivore Irena puella, and an understorey insectivore, Pellorneum tickelli. Other common species included Macronous gularis, Pycnonotus melanicterus, and Yuhina zantholeuca (89% of lists); Hypothymis azurea and Orthotomus sutorius (67% of lists). The Khiriwong forest patch was less diverse than Krung Ching and somewhat similar in diversity and dominance to the orchards (Appendix 1). While it held some forest species that were absent from the orchards, such as Irena puella, Terpsiphone paradisi, and the Sundaic babblers Malacopteron magnirostre and Alcippe brunneicauda, it lacked the suite of lowland forest species recorded from Krung Ching. Seven species of babblers (Timaliidae) contributed 17% of registrations in the forest patch. Although this exceeded the diversity in the orchards it fell far short of the 13 babbler species at Krung Ching. The only babbler species added by the Khiriwong forest patch that was not found elsewhere, and incidentally also the commonest, was the submontane and montane Pellorneum tickelli. More than 75% of all babbler registrations in the Khiriwong forest patch were contributed by this species, together with the very tolerant and wide-ranging Macronous gularis and Yuhina zantholeuca. Overall, the proportion of total registrations contributed by Sundaic bird species was the same as that in the orchards (26%), only half that in the Krung Ching lowland forest (below). No hornbills or any other nationally rare or threatened species were detected in the Khiriwong forest patch. Krung Ching In comparison with both Khiriwong plots, the commonest species at Krung Ching showed less overall predominance, as would be expected of a more diverse habitat. No species occurred on 100% of lists (Appendix 1). Larger frugivores were frequent. In contrast to the orchard, where just one species of barbet, Megalaima sp., was recorded, Krung Ching held four species, all relatively common. One of these, Megalaima mystacophanos, was among the commonest species on the plot, being recorded on 73% of lists. Another frugivore, Irena puella, was even more frequent (82% of lists). A great range of arboreal and understorey insectivores was also present. The commonest bulbul found at Krung Ching, Alophoixus bres (64% of lists), was characteristic of forest at lower elevations and was recorded in neither of the Khiriwong plots but was replaced by A. ochraceus in the forest patch (Appendix 1). Ecotone-inhabiting bulbuls, and those feeding partly on the small fruits of pioneer tree species (e.g. most Pycnonotus spp., Iole olivacea), which predominated in the Khiriwong orchard, were scarcer at Krung Ching. Krung Ching held 13 species of babblers compared with three species in the Khiriwong orchard and seven in the forest patch. None of the three commonest babblers at Krung Ching, the two foliage gleaners, Stachyris erythroptera and Malacopteron cinereum, and the terrestrial Pellorneum capistratum was recorded at the other two sites. Species of Sundaic affinity, most of which are obligate forest birds (Wells 1976), contributed 122 of 220 bird registrations at Krung Ching (55.5%), more than twice the proportion in the other sites. Approximately 19 species recorded at Krung Chung (23%) would probably not be expected to occur higher than 500 m elevation, based on their (lowland forest) habitat requirements. Even so, the Krung Ching sample should not be taken as fully representative of a lowland forest community since, even there, the plains were already devoid of forest cover, and many extreme lowland specialists were not detected. The presence of four species of hornbills at Krung Ching, and their complete absence from the other two sites was especially noteworthy. Eight species of bird found at Krung Ching were listed as nationally threatened or nearthreatened by Round (2000): in addition to the hornbills mentioned above, these were Indicator archipelagicus, Oriolus xanthonotus, Trichastoma bicolor and Stachyris maculata. Krung Ching also still supported some arboreal mammals. Both White-handed Gibbon Hylobates lar and Dusky Leaf Monkey Trachypithecus obscurus were heard, and a few Black Giant Squirrels Ratufa bicolor were seen. None of these species was recorded at either of the Khiriwong sites. Human disturbance Although this survey did not attempt a detailed examination of use of forest and forest products by local people, this is clearly an aspect of crucial importance in understanding local avifaunas and other aspects of biodiversity. The effects of human use are varied, impacting on the avifauna both directly (hunting and persecution) and indirectly (through habitat loss and fragmentation). Hunting and trapping The local people at Khiriwong were still trapping wild birds in violation of wildlife protection laws. On 22 November 2001 we observed a group of men walking towards Khiriwong village with two cages, each containing a Blue-winged Leafbird. These could have been newly trapped birds or live decoys used to attract birds into traps. Eleven Irena puella, 10 Copsychus malabaricus, one Loriculus vernalis, one Red-whiskered Bulbul Pycnonotus jocosus, one Verditer Flycatcher Eumyias thalassina, and one unidentified blue flycatcher Cyornis sp. were observed in cages in Khiriwong village. The absence of shamas in both orchards and forest fragment was almost certainly due at least partly to trapping. Larger species such as hornbills are particularly vulnerable to poaching and collection of nestlings for the pet trade. It is likely that most hornbills were eradicated from Khiriwong many years ago due to a combination of direct disturbance and habitat loss. Removal of natural forest cover The most obvious human disturbance at Khiriwong was the loss of natural forest cover from most areas below 600–700 m elevation which removed habitat for a wide range of the more ecologically sensitive birds. The Khiriwong forest patch, the lowest we could find in the immediate vicinity of the orchards, lay well above the normal upper altitudinal limits for some species found at Krung Ching, such as Oriolus xanthonotus, Trichastoma bicolor, and Stachyris maculata. Despite assertions about the stability of the agroforestry systems at Khiriwong, the replacement of forest by plantation was still in progress, and gradually encroaching up the slopes of Khao Luang National Park from 700 m to at least 800 m elevation. Gradual enlargement of cultivated areas is a persistent problem in situations where local people are farming land in close proximity to forest: it is very difficult to monitor because the boundary between forest and cultivated land is seldom surveyed and marked. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DISCUSSION All three sites surveyed showed a moderate to high diversity of birds. Other than for vegetation type, the two lowland/foothills sites, Khiriwong orchardand Krung Ching, were judged to be comparable in their environmental features and location. Both covered a roughly similar range of elevations and included major streams (though Krung Ching had somewhat less steep topography than Khiriwong). Khiriwong was surveyed during October, after the end of the breeding season, when bird vocalising was generally at a lower level, whereas Krung Ching was censused in April, at a time when most forest birds were breeding and probably vocalised more. However, this was not thought to be a significant source of error, since birds were detected both by sight and by sound. It probably takes longer to amass a sample when birds are calling less, but there is little bias over differential detectability among species using MacKinnon Lists (O’Dea et al. 2004). The main exception for this are the parasitic cuckoos, Cuculus micropterus, Cacomantis spp., Chrysococcyx spp., and Surniculus lugubris, which are relatively difficult to observe, but have loud vocalisations during the breeding season. However, parasitic cuckoos contributed only 3 of 220 registrations at Krung Ching, too small a proportion to pose any significant bias. Moreover, a full complement of wintering migrants was believed present during all survey periods. The three sites were strongly dissimilar, with rather limited overlap among their avifaunas. The Khiriwong orchard differed markedly in the structure and composition of its bird community in comparison to the lowland forest, as exemplified by Krung Ching, within the adjacent national park. In particular, the orchards lacked most of those species characteristic of the Sundaic lowland forest bird community, and the majority of species recorded were widespread and ecologically tolerant, and found in a range of habitats including forest, forest edge, plantations or open cultivated land throughout Thailand. Babblers (Timaliidae) are generally good indicators of diversity, since all are shortwinged forms with rather limited dispersal capabilities. The larger, least disturbed forests are rich in babblers. For example, Khao Nor Chuchi, possibly the single richest lowland forest site remaining in southern Thailand, possesses at least 22 species of babblers among the 318 bird species recorded there (Round and Treesucon 1998). The Khiriwong orchards were especially depauperate in understorey-inhabiting insectivores such as babblers, and even the relatively ecologically tolerant Copsychus malabaricus, while a great many other species of obligate forest bird found at Krung Ching were also absent. Although smaller frugivores and nectarivores fared better, they were heavily biased in favour of edge-inhabiting species. Other, larger, frugivores that were abundant in forest, such as Irena puella, were absent. Our results closely parallel those of Thiollay (1995) for Indonesian traditional agro-forests. Bird species richness in various Indonesian agroforests was about one-third to slightly more than one-half that in primary forest. Larger frugivores, larger insectivores of both canopy and understorey, and terrestrial insectivores of the forest interior were absent from or much reduced in agroforests, while species persisting included small frugivores, smaller foliage gleaning insectivores, nectarivores, and edge species. Fully 41% of birds encountered in forest were never found in the agro-forest, while 25% of the bird species in agro-forest did not enter forest (Thiollay 1995). Disturbance of natural vegetation did not merely cause a reduction in number of bird species but also involved replacements of the more sensitive forest-living species by others more tolerant of disturbance. Although the Khiriwong forest patch was surrounded by orchards and only a short distance from the orchard surveyed, it differed markedly in the composition of its avifauna, possessing a few more forest species, and a few ‘new’ species characteristic of higher elevations. Nonetheless, it bore more similarity to the orchard than it did to the other closed canopy forest site. Some edge-inhabiting species may move between the orchards and the forest patch, while many forestinterior species were probably lost from the forest patch due to area effects. The smallness of the forest patch may cause it to mirror the attributes of surrounding cultivated areas to some degree (cf. Diamond et al. 1987). It was clearly too small to hold larger birds which require large home ranges, such as hornbills (Poonswad and Tsuji 1994). All four hornbill species found at Krung Ching occur elsewhere in southern Thailand at elevations comparable to that of the Khiriwong forest patch. However, it is not easy to disentangle the effects of fragmentation from other factors (McGarigal and Cushman 2002). Hornbills will also have been impacted by hunting, the effects of which are liable to be more severe in small forest patches, where there is little, or reduced, possibility of recolonisation from elsewhere. Habitat fragmentation also impacts on smaller birds. The forest interior frugivore Calyptomena viridis was not detected in the forest patch. Ford and Davison (1995) also noted a marked reduction in babblers and other understory birds even from patches as large as 500–800 ha. Mixed-species forest orchards such as those at Khiriwong offer food resources for a number of smaller nectarivorous and frugivorous birds, such as Megalaima australis, Loriculus vernalis, Dicaeum trigonostigma and Arachnothera chrysogenys, and also a few insectivores (including, in this case, three species of woodpeckers, Celeus brachyurus, Picus miniaceus and Meiglyptes tristis) that would be unlikely to occur in monoculture plantations such as rubber. The species accumulation curve for rubber plantation surveyed at Khao Nor Chuchi, Krabi, S. Thailand, by Gro-Nielsen (1997, unpublished report) reached an asymptote at £ 25 species, less than half that for Khiriwong. Khiriwong was therefore diverse in comparison with other agricultural areas where rubber plantations have been allowed to ascend hill-slopes to 600 m or higher. The Khiriwong model might, therefore, be appropriate for habitat diversification, and restoration of modest levels of biodiversity in sites that have already been heavily impacted by man, and areas committed to agricultural use. However, it is certainly not a satisfactory alternative to conservation forest in protected areas where exclusion of human exploitation is the best means of maintaining biodiversity. Plant species richness will impact bird diversity since a plant community that is poorer in species will, other things being equal, offer fewer foraging niches for birds (Wiens and Rotenberry 1981; Hansen et al. 1995). Clearly, the Khiriwong orchards had many fewer species of trees than the forest areas and 2883 it would be expected that orchards offer a smaller range of fruit and other food resources than forest. However, it is unclear to what extent the poor representation of understorey and middle storey insectivores is a function of reduced structural complexity, as opposed to biotic complexity, of the vegetation. The forests had a greater density of vegetation occupying the lower strata (1–6 m), whereas the understorey in the Khiriwong orchards was sparse. It is possible that if more varied undergrowth was allowed to grow between and beneath the fruit trees, populations of some understorey birds might eventually recover. Mitra and Sheldon (1993) found a relatively diverse insectivorous bird fauna inhabiting the understorey and middle storey of plantations of the exotic tree Albizia falcataria in Borneo. By default, the model for development followed in southern Thailand and elsewhere throughout the Sunda subregion has been one in which rural populations have been allowed to expand cultivation to occupy virtually the entire lowland area, with forest being allowed to remain, if at all, on hill-slopes only. While this may be appropriate for upper watershed conservation, it is a very poor strategy for biodiversity conservation. As this study has shown, neither patches of closed canopy, multi-storeyed forest on hill-slopes nor tall, mature orchards of the valley-bottom and foothills can compare with lowland forest in terms of avian species richness. These findings are highly significant for pursuing biodiversity conservation in man-modified landscapes. We should not be seeking, as a general principle, to extend or legitimise extractive human use in national parks and other protected areas, as many Thai NGOs, and some international conservation agencies (ICEM 2003), have suggested, since this would further reduce biodiversity. A more appropriate strategy would be to encourage agricultural diversification in existing plantations, while seeking to restore original forest cover in selected areas around the lowland margins of existing protected areas, perhaps through ‘buy-back’ or financial compensation to farmers or landowners, such as has been implemented in (e.g.) Florida, USA (Main et al. 1999). |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ACKNOWLEDGEMENTS We thank Mr. Sathien Chuaynoo, Superintendent of Khao Luang National Park, Ms. Somjit Huangdilok, National Parks Division, Department of National Parks, Wildlife and Plants Conservation, Bangkok; Mr. Therdthai Khwangthong, head of the Krung Ching Guard Station, and the villagers of Ban Khiriwong for their assistance and hospitality. PDR would also like to thank Ms. Sukanya Thanomphut and Dr. Panom Archarit for facilitating his visit to Krung Ching. Dr. John Milne, John Parr and Dr. Sompoad Srikosamatara commented on versions of this manuscript. This survey was conducted in collaboration with the National Center for Genetic Engineering and Biotechnology, Bangkok, Thailand, with support from the Biodiversity Research and Training Program, Grant BRT 244003. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Appendix 1 : Numbers and species of birds recorded in orchard and forest habitats, Khao Luang National Park.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Numbers represent no. of occurrences on 20-species MacKinnon Lists. (Khiriwong orchard, 10 lists; Khiriwong forest patch, 9 lists; Krung Ching, 11 lists). Feeding guilds assigned after Johns (1986): TF, terrestrial frugivore; AF, arboreal frugivore; FF, arboreal faunivore/frugivore; TIF, terrestrial insectivore/ frugivore; AIF, arboreal insectivore/frugivore; IN, insectivore/nectarivore; TI, terrestrial insectivore; BGI, bark-gleaning insectivore; FGI, foliage-gleaning insectivore; SaI, Sallying insectivore; SwI, sweeping insectivore (exclusively aerial feeder); R, raptor; P, piscivore. Species of mainly or entirely Sundaic distribution are shown in bold type. (There appear to be no previous records of D. hottentottus for the Thai–Malay Peninsula. This record is provisionally retained for the purposes of this analysis, while recognising that further evidence would be necessary before adding it to the faunal list. D. h. brevirostris is a common winter visitor to a range of woodland habitats, including close canopy evergreen forest in continental Thailand and its occasional occurrence in peninsular Thailand seems likely.) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| REFERENCES Bibby C., Jones M. and Marsden S. 1998. Expedition Field Techniques: Bird Surveys. Royal Geographical Society, London. Bruner A.G., Gullison R.E., Rice R.E. and da Fonseca G.A.B. 2001. Effectiveness of parks in protecting tropical biodiversity. Science 291: 125–128. Colwell R.K. 2004. EstimateS: Statistical estimation of species richness and shared species from samples. Version 7. User’s Guide and application published at http://purl.oclc.org/estimates. Diamond J.M., Bishop K.D. and van Balen S. 1987. Bird survival in an isolated Javan Woodland: island or mirror? Conserv. Biol. 1(2): 132–142. Fisher R.J. 1995. Collaborative Management of Forests for Conservation and Development. IUCN/WWF, Gland, Switzerland. Ford H.A. and Davison G.W.H. 1995. Forest avifauna of Universiti Kebangsaan Malaysia and some other forest remnants in Selangor, Peninsular Malaysia. Malay. Nat. J. 49: 117–138. Ghimire K.B. and Pimbert M.P. (eds) 1997. Social Change and Conservation: Environmental Politics and Impacts of National Parks and Protected Areas Earthscan, London. Gujral R.S. 1991. Types of agroforestry systems in Asia-Pacific region. In: Mellink W., Rao Y.S. and MacDicken K.G. (eds), Agroforestry in Asia and the Pacific. RAPA Publication 1991/5. Regional Office for Asia and the Pacific (RAPA), FAO, Bangkok, Thailand, pp. 195–201. Hansen A.J., McComb W.C., Vega R., Raphael M.G. and Hunter M. 1995. Bird–habitat relationships in natural and managed forests in the west cascades of Oregon. Ecol. Appl. 5: 555–569. ICEM 2003. Regional Report on Protected Areas and Development. Review of Protected Areas and Development in the Lower Mekong River Region. Indooroopilly, Queensland, Australia. Juiprik S. 1997. Suan Somrom: Watthanatham Khon Phaktai. Forest Commun. Newslett. 4(7): 27–37. Regional Community Forestry Training Center, Bangkok. (in Thai). Johns A.D. 1986. Effects of selective logging on the ecological organization of a peninsular Malaysian rainforest avifauna. Forktail 1: 65–79. Leigh E.G. Jr. and de Lao S.L. 2000. Fishers’ alpha: measuring tree diversity. Inside CTFS, Summer 2000 12: 6–7. Lekagul B. and Round P.D. 1991. A Guide to the Birds of Thailand. Saha Karn Bhaet, Bangkok. MacKinnon J. and Phillipps K. 1993. A Field Guide to the Birds of Sumatra, Java, and Bali. Oxford University Press, Oxford. Main M.B., Roka F.M. and Noss R.F. 1999. Evaluating costs of conservation. Conserv. Biol. 13: 1262–1272. Makarabhirom P. 1991. Agroforestry in Thailand. In: Mellink W., Rao Y.S. and MacDicken K.G. (eds), Agroforestry in Asia and the Pacific. RAPA. Publication 1991/5. Regional Office for Asia and the Pacific (RAPA), FAO, Bangkok, Thailand, pp. 168–181. Makarabhirorm P. 1998. The Thailand Community Forestry Bill: the Ongoing Debate. Asia- Pacific Commun. Forest. Newslett. 11(1): 14–17. McGarigal K. and Cushman S.A. 2002. Comparative evaluation of experimental approaches to the study of habitat fragmentation effects. Ecol. Appl. 12: 335–345. Michon G. and de Foresta H. 1990. Complex agroforestry systems and conservation of biological diversity. In: Kheong Y.S. and Win L.S. (eds), Harmony with Nature: Proceedings of the International Conference on Conservation of Tropical Biodiversity, 12–16 June 1990. Malayan Nature Society, Kuala Lumpur, pp. 457–473. Mitra S.S. and Sheldon F.H. 1993. Use of an exotic tree plantation by Bornean lowland forest birds. Auk 110(3): 529–540 O’Dea N., Watson J.E.M. and Whittaker R.J. 2004. Rapid assessment in conservation research: a critique of avifaunal assessment techniques illustrated by Ecuadorian and Madagascan case study data. Divers. Distrib. 10: 55–63. Poonswad P. and Tsuji A. 1994. Ranges of males of the Great Hornbill Buceros bicornis, Brown Hornbill Ptilolaemus tickelli and Wreathed Hornbill Rhyticeros undulates in Khao Yai National Park, Thailand. Ibis 136: 79–86. Ramitanondh S. 1989. Forests and deforestation in Thailand: a pandisciplinary approach. In: Thailand: A Symposium of the Siam Society. The Siam Society, Bangkok, pp. 23–48. Round P.D. 1988. Resident Forest Birds in Thailand: Their Status and Conservation. ICBP Monograph No. 2. International Council for Bird Preservation, Cambridge, UK. Round P.D. 1998. Wildlife, habitats and priorities for conservation in Dong Khanthung proposed NBCA, Champasak Province, Lao PDR. Final Report. CPAWM and Wildlife Conservation Society, Vientiane. Round P.D. 1999. Avifaunal surveys of the Pu Mat Nature Reserve, Nghe An Province, Vietnam, 1998–1999. Final Report. Social Forestry and Nature Conservation in Nghe An Province. ALA/ VIE/94/24. Ministry of Agriculture and Rural Development and the European Commission, Vinh, Vietnam. Round P.D. 2000. Field Check-List of Thai Birds. Bird Conservation Society of Thailand, Bangkok. Round P.D. and Treesucon U. 1998. Birds of Khao Nor Chuchi: Check-List and Guide to Bird Finding. Bird Conservation Society of Thailand, Bangkok. Salafsky N., Cauley H., Balachander G., Cordes B., Parks J., Margoluis C., Bhatt S., Encarnacion C., Russell D. and Margoluis R. 2001. A systematic test of an enterprise strategy for communitybased biodiversity conservation. Conserv. Biol. 15: 1585–1595. Scott P. 1998. From Conflict to Collaboration: People and Forests at Mount Elgon, Uganda. IUCN, Gland, Switzerland and Cambridge, UK. Thiollay J.-M. 1995. The role of traditional agroforests in the conservation of rain forest bird diversity in Sumatra. Conserv. Biol. 9(2): 335–353. Tourism Authority of Thailand. 2000. Adventure Destinations Nakhon Si Thammarat. 16 September 2002, <http://www.tat.or.th/do/nakorn-sri.htm>. Wells D.R. 1976. Resident birds. In: Medway L. and Wells D.R. (eds), The Birds of the Malay Peninsula. Vol. 5. Witherby and Penerbit Universiti Malaya, London, pp. 1–33. Wiens J.A. and Rotenberry J.T. 1981. Habitat associations and community structure of birds in shrub steppe environments. Ecol. Monogr. 51(1): 21–42. Wiersum K.F. 1991. Promoting agroforestry: framework for a national action plan. In: Mellink W., Rao Y.S. and MacDicken K.G. (eds), Agroforestry in Asia and the Pacific. RAPA Publication 1991/5. Regional Office for Asia and the Pacific (RAPA), FAO, Bangkok, Thailand, pp. 202–218. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kindly
submitted by: *Philip D. Round, Department of Biology, Faculty of Science, Mahidol University, Rama 6 Road, Bangkok 10400. E-mail: frpdr@mahidol.ac.th George A. Gale, School of Bioresources and Technology, King Mongkut’s University of Technology Thonburi, Bangkhunthien, Bangkok 10150 Warren Y. Brockelman, Department of Biology, Faculty of Science, Mahidol University, Rama 6 Road, Bangkok 10400 *Author to whom correspondence should be sent. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tweet | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

A Guide to Birdwatching in Thailand. Copyright © 2004-2017 thaibirding.com. All rights reserved.