
|
||||||||||
|
||||||||||
| Favorite Tweets by @thaibirding | ||||||||||
|
||||||||||
 Buy Birds of Thailand on Amazon.co.uk |
||||||||||
| Site Map ; Contributors |
| Changes
in the Status of Lophura Pheasants in Khao Yai National Park, Thailand: A Response to Warming Climate? By Philip D. Round & George A. Gale |
| Tweet |
| Note: This article was originally published in BIOTROPICA 40 (2), 2008 and was kindly submitted by Philip D. Round. |
| INTRODUCTION Over the past 100 years, the global average temperature has increased by approximately 0.6 degrees C and is expected to continue to rise rapidly (Houghton et al. 2001). Although species have responded to climatic changes on an evolutionary timescale, a critical question for wild species is how they cope with the current rate of change (Root et al. 2003). Among birds, climate change is linked with earlier breeding (Crick et al. 1997, Forcchammer et al. 1998, McCleery & Perrins 1998, Crick & Sparks 1999, Schaefer et al. 2006), earlier arrival dates, and later departure dates in breeding visitors (Mason 1995, Butler 2003, Lehikoinen et al. 2004, Beaumont et al. 2006, Jonz´en et al. 2006). Poleward and altitudinal range expansions have been documented in birds and in many other taxa (Thomas & Lennon 1999, Hickling et al. 2006). Typically, studies of responses of birds and other species to climate change have focused on organisms occurring in the temperate zone and climatic zones closer to the poles (Root et al. 2003).However, since the mid- 1970s all tropical rain forest regions have experienced a significant warming at a mean rate of 0.26 ± 0.05 degrees C per decade, in synchrony with the global increase (Malhi & Wright 2005). In one of the few tropical studies on birds, Pounds et al. (1999) documented rapid change in the avifauna (the colonization of montane habitats by nonmontane species) linked to declining mist frequency (itself induced by rising air temperatures) in cloud forest in Costa Rica. Here, we investigate whether the incidence of two species of Lophura pheasants that occur syntopically in a mid-elevation forest in Khao Yai National Park, northeastern Thailand has changed in the past two to three decades, and speculate on the possible causes. The Silver Pheasant Lophura nycthemera and Siamese Fireback L. diardi occur sympatrically over much of their SE Asian ranges encompassing north and east Myanmar, northern and eastern Thailand, and Indochina (King et al. 1975, Delacour 1977, Dickinson 2003). The race of Silver Pheasant occurring in northern Thailand, south to Khao Yai, L. n. jonesi, is mainly montane and submontane in distribution, inhabiting elevations of 700 m and above in Thailand, though other races are occasionally found lower, down to 200 m in some parts of Laos and Vietnam, (J. W. Duckworth, pers. comm., J. C. Eames, pers. comm., R. Timmins, pers. comm.). The monotypic L. diardi is a lowland species, in Thailand mainly inhabiting forest in plains and foothills to a maximum elevation of 700–800 m (King et al. 1975, Lekagul & Round 1991, Robson 2000), though usually found below 500 m in Laos (J. W. Duckworth, pers. comm.). Although there is substantial local variation in altitudinal range in both species across their ranges, the two are only rarely found alongside each other in Laos and Vietnam (J. W. Duckworth, pers. comm., J. C. Eames, pers. comm.). In their relatively wide zone of geographical overlap, the two species are thus largely segregated by habitat and elevation. |
| Methods STUDY AREA - Khao Yai is Thailand’s oldest park, established in 1962, and is occupied mainly by evergreen or semi-evergreen forest, with small areas of mixed deciduous forest around the northern margins. Much of this forest is tall and mature, with large trees, and is roughly evenly divided between foothills (< 600 m) and submontane elevations (600–1000 m). However, significant areas in the foothills zone, such as along the southern access road from the headquarters descending to the town of Prachinburi, have been logged. Observations were collected within a radius of 5 km of the park headquarters.Most of the area was evergreen forest alternating with patches of Imperata-dominated grassland, left over from former cultivation, the area having supported a small human population before the park was established. Parts of this grassland area are maintained as grazing habitat for large herbivores by annual burning or mowing. The topography is an undulating plateau, at elevations 600–890 m, with over 80 percent of the area lying > 750 m. The plateau is transected by a major stream, the Lam Takhong, and there are many smaller streams, most of which dry up seasonally during February to April. The area has been regarded as lying in an altitudinal transition zone between lowland forest and hillslope (or even lower montane) forest (e.g., Smitinand 1968). The highest summit in the park, Khao Rom (1350 m), lies only 8.5 km SE of the study area. RECORDS OF LOPHURA PHEASANTS IN KHAO YAI. - Dickinson (1963) was the first to record L. nycthemera in Khao Yai National Park, though Deignan (1963) was presumably aware of its occurrence there, since he listed the species ‘south as far as Nakhon Ratchasima,’ the province in which a major part of Khao Yai lies.McClure (1974) listed both L. nycthemera and L. diardi for Khao Yai but described both as rare, and his seasonal distribution table provides only one monthly entry for each species, indicating that he made, or knew of, very few sightings, perhaps as few as one of each species, during the period of his coverage (1967–1973). Notwithstanding the scarcity of early records, both L. nycthemera and L. diardi are now known to be relatively common, and are frequently observed in the headquarters area of Khao Yai. SOURCES AND ANALYSES OF DATA.—Sightings of both Lophura pheasants were usually reported in the written accounts of birdwatchers, filed at the (now defunct) Association for the Conservation of Wildlife, Bangkok, during 1978–1986, and at either the Center for Conservation Biology,Mahidol University, Bangkok, or the Bird Conservation Society of Thailand from 1986 onwards up to the present, and this constituted our primary data source. Further, an appeal for all sightings of either L. nycthemera or L. diardi in Khao Yai was posted on the Oriental Birding Newsgroup in January 2000. The data supplied were usually numbers and sexes of pheasants sighted, date and duration of visits, and approximate location (by trail), and were therefore amenable to analysis, even though the search effort was not standardized. In addition, we incorporated all our own sightings, made both while birdwatching in the park, and implementing standardized surveys (below). The extensive trail and road network allowedmore or less even access across the limited range of elevations within the park headquarters area. We noted all records of Lophura pheasants, and recorded search effort as number of days per trip, regardless of the number of observers. Negative records were also included. Simple linear correlations were used to assess changes in pheasant encounter rates and changes in rainfall and temperature through time. Distance data from line transects conducted on a mapped 30-ha plot, the Mo-Singto Long-term Biodiversity Study Plot, lying 800 m from the park headquarters, during May 2003 to August 2005, analyzed using DISTANCE 5.0 software (Thomas et al. 2004), were employed to estimate densities and probability of detections of both species. Results Records (not including those from transect sampling) of a total of 354 individual pheasants were available for the period of analysis. This comprised 84 sightings (177 individuals) of L. diardi and 87 sightings of 177 individuals of L. nycthemera. Eighty-six individuals of both species combined were seen during the 15-yr period from 1979 to 1993 compared with 268 individuals during 1994–2004 inclusive (Fig. 1). The approximate detection rates for these two periods, for both pheasants combined, were 0.21 pheasants per day and 0.67 pheasants per day, respectively. |
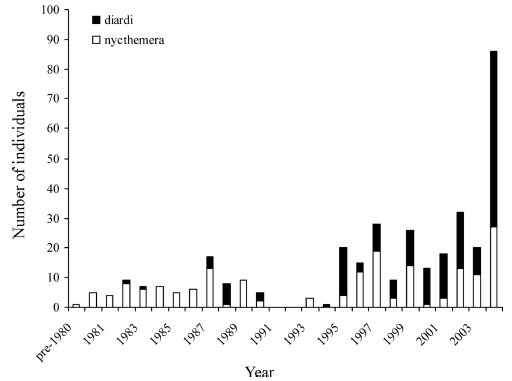 Figure 1: Numbers of Lophura pheasants detected in Khao Yai National Park, ca 1978–2004. |
| The
proportions of sightings contributed by each species varied markedly
through time. Up to 1993, L. diardi contributed only 16
of 86 individual pheasants seen (18.6%) with only two sightings
of single individuals being made before 1987. In the period from
1994 to the present, L. diardi contributed more than half of all
pheasants observed (60.1%; 161 out of 268 observations). Lophura
diardi contributed a significantly higher proportion, and L.
nycthemera a significantly lower proportion, of pheasants observed
in the second period than in the first (?2 = 43.1; P < 0.01,
df = 1). There was a significant increase in the detection rate of L. diardi during the survey period, both as measured by the number of individuals per day (r = 0.607; P = 0.002), and the number of sightings per day (r=0.686; P<0.001; Fig. 2). In contrast, neither the number of individuals detected nor the number of sightings per day increased significantly in L. nycthemera (r = 0.164, P = 0.45 and r = 0.265, P = 0.708, respectively). There was no significant increase in flock size in either species during the study period (L. nycthemera mean flock size: 1.98 ± SD 1.42; r = 0.095, P = 0.669; L. diardi mean flock-size: 2.22 ± 1.44; r = 0.376, P = 0.152). |
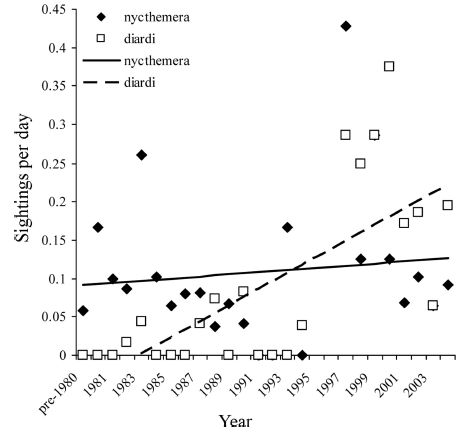 Figure 2: Detection rates of Lophura pheasants, 1980–2004. |
| The
disparity between the detection patterns of L. nycthemera
and L. diardi was very striking. One of us (PDR) spent
many hours in 1982–1985 walking trails on the 30-ha Mo-Singto
study plot, with access routes to and from the plot probably adding
another 50 ha in total. Though L. nycthemera was seen eight
times during that period there were no sightings of L. diardi.
A colleague spent approximately 20 d/mo during the period November
1981 to March 1983 studying White-handed Gibbons Hylobates lar
on the same plot, encountering L. nycthemera at least six
times during that period, but never once seeing L. diardi (U.
Treesucon, pers. comm.). At the present time, the majority of pheasant encounters on and around the Mo-Singto plot are with L. diardi. During 2001–2004 we recorded 23 sightings of 48 individual L. ycthemera, compared with 49 sightings of 84 L. diardi on, or in the immediate vicinity of, the study plot. Camera-trapping, conducted beneath fruiting Prunus trees during 2004 recorded ca. eight L. diardi compared with only one L. nycthemera (W.Y. Brockelman, pers. comm.). The latter observer, who has worked in the park for nearly 30 yr, commented that L. nycthemera ‘used to be much more common’ (W.Y. Brockelman, pers. obs.). During line transect surveys, we recorded 13 sightings of 23 adult L. nycthemera and 15 sightings of 34 adult L. diardi. Only six records of L. nycthemera (46.1%) were on lower, flatter areas below the 770-m contour, while the 11 of 15 L. diardi records (73.3%) were below this contour. However this difference was not statistically significant (?2 = 2.23, P = 0.142) and there was much overlap. For density estimation, due to the small sample size, and as detection distances between species appeared to be similar, data from both specieswere pooled to generate a single detection function following Buckland et al. (2001). The density estimate for L. diardi was greater (10.0 groups or 22.8 individuals/km2, vs. 8.0 groups or 14.7 individuals/km2) However, due to the small sample size, the variances of the estimates were large (95% CI: 4.7–21.5 groups/km2 for L. diardi and 2.6–24.5 groups/km2 for L. nycthemera) suggesting that the estimates should be treated with caution (Buckland et al. 2001). Discussion Both Lophura pheasants presently co-exist in the headquarters of Khao Yai, though their relative abundance has changed markedly over the past two decades. The proportion of sightings contributed by L. diardi changed from 18.6 percent during 1980–1993 (all but two of these sightings coming from 1987 to 1993) to 60.1 percent during 1994 to 2004. There was a significant increase in the rate of detections (sightings/unit effort) of L. diardi throughout the study period, whereas the detection rate of L. nycthemera remained unchanged. Although observers identified most sightings by trail, most sightings could not be related to point locations.Data collection was not standardized, though there was no bias toward data collection from higher or lower elevations, or from differential spatial coverage during any period of the study that could account for the observed change in incidence of the two species through time. While it is likely that some of the same individuals were observed in the same locations repeatedly by different observers, the change in the relative proportion of observations of one species to another would not be expected to show a strong bias over time. Some of the increase in sightings may have been due to pheasants becoming tamer and easier to observe than formerly, following the cessation of hunting in the 40 yr since the park was established. The improved skills and more detailed knowledge of birdwatchers on where to find pheasants within the park may also have contributed. Much less information was available pre-1990. Neither increased habituation nor differences in coverage can account for the changing proportions of the two species, however. If one or the other species had been more adversely affected by, or less able to rapidly recover from, predation, this might lead to differential detectability though time. However, there is no evidence that either was the case. Both species are equally targeted by hunters. Clutch sizes are also similar (reported as 6–8 eggs for L. n. jonesi and 4–8 eggs for L. diardi; A. Hennache, pers. comm. to P. Garson) suggesting no major difference in intrinsic rates of increase. Captive L. nycthemera maintained in (Thai) Department of National Parks breeding stations produced slightly more eggs than did L. diardi (average 19.4 ggs/female/yr in L. nycthemera compared with 18.4 eggs in L. diardi (P. Sanpote, pers. comm.). There are no data on juvenile survival, which is likely to be more important than clutch size in affecting recruitment, but again is unlikely to have created such an obvious change in detections. There has been no major change in habitat, such as the breakdown in a former physical or vegetational barrier, which could account for a rapid colonization of the headquarters area by a species that was formerly absent. The southern parts of Khao Yai slope down gently from > 1000 m to the park boundary to ca 100 m, and in most of the area the forest cover is unbroken. Although a northern access road into the park road was constructed during the late 1950s and early 1960s, and a connecting road to the southern park boundary was built in 1982, this low level of habitat perturbation would not have affected the distribution of forest interior species such as pheasants. A few open-country or deciduous woodland birds that have apparently benefited, and have recently colonized the headquarters area of the park (e.g., Asian koel Eudynamys scolopaceus, plaintive cuckoo Cacomantis merulinus, red-wattled lapwing Vanellus indicus, racket-tailed treepie Crypsirina temia, common myna Acridotheres tristis and olive-backed sunbird Nectarinia jugularis; Lynam et al. 2006) are all confined to forest edge or grassland, and their numbers have remained small. Nor is it likely that loss of habitat in the lowlands has displaced L. diardi and causedmore individuals tomove upslope into suboptimal habitat formerly occupied solely by L. nycthemera. If this was the case, it would be likely that, after some initial increase in numbers in that habitat, the population would gradually dwindle as senescent birds, unable to sustain their numbers through recruitment, died off. SYNTOPY AMONG LOPHURA PHEASANTS. - Why has L. diardi recently become more detectable in relation to L. nycthemera? One should first consider how usual is the co-existence of two Lophura species in the same habitat. In the few cases where Lophura pheasants occur in sympatry, they are usually ecologically segregated by habitat or elevation (Davison 1981; Thewlis et al. 1998; BirdLife International 2000, 2001). The co-occurrence of two Lophura in lowland mixed dipterocarp forest of Malaysia (Davison 1981) occurs in the context of an exceedingly rich avifauna and a high level of endemism at the Sunda subregional level. The avifauna of Khao Yai, by contrast, is relatively species-poor. It supports, for example, only ten species of babblers and laughingthrushes (Timaliiinae and Garrulacinae) compared with roughly 25 species of babblers in lowland Sundaic rain forest (Wells 1985). This makes it all the more surprising that two Lophura pheasants should both be relatively common in the same habitat. The present syntopy of two congeneric pheasants in Khao Yai’s relatively depauperate avifauna is unlikely to reflect a stable situation, but instead indicates a dynamic interaction, in which the lowland species, L. diardi, is increasingly moving into the habitat of the higher elevation species, L. nycthemera. Even thoughboth species seemingly take a wide range of plant matter and invertebrates, the two overlap greatly in feeding ecology, and an increase in one species might be expected to affect the numbers of the other. POSSIBLE EFFECTS OF CHANGING CLIMATE. - The most likely reason for an increase in numbers of L. diardi is the operation of an environmental factor that favors this species. We suggest that factors mediated by changing climate are the most plausible explanation for the increased frequency of sightings of L. diardi. Ecological changes caused directly by rising temperature, or mediated through increased evapotranspiration, would be expected to benefit plants and animals of drier lowland habitats at the expense of montane species. Logically, one might assume that animals would track vegetational changes.However, changes in distributions might be detected more easily in animals than in plants due to their greater conspicuousness.Moreover, animals could be responding to minor vegetational changes such as those demonstrated by Karr and Freemark (1983) in the understory of a Panamanian forest including changes in leaf litter depth, soil humidity or other microclimatic features. Detailed mapping of the forest tree flora on the Mo-Singto permanent plot, carried out over several years (Brockelman et al. 2002), provides indications that changes in seedling recruitment among forest trees are taking place. Seedlings of the third most common tree on the plot (Nephelium melliferum Fam. Sapindaceae) are failing to survive on south and west-facing slopes, while adult trees are distributed throughout the plot. This has tentatively been attributed to rising temperatures (Brockelman et al. 2005). The direct evidence for temperature change at Khao Yai is equivocal, however. Temperature graphs for the two largest provinces abutting Khao Yai, Nakhon Ratchasima to the north and Prachinburi to the south, show a significant rising trend in two of three measurements. The mean minimum and mean temperature for Nakhon Ratchasima, (r = 0.855, P < 0.001 and r = 0.840, P < 0.001, respectively) and the mean minimum and mean maximum for Prachinburi (r = 0.713, P < 0.001 and r = 0.706, P<0.001, respectively) increased by 1.5–2.0?C over a 50-yr period (data supplied by Meteorology Department, Bangkok). Although the trends differ between the two stations for mean maximum and mean temperatures, the trend of increase for mean minimum temperature was very similar in both. However, the rise documented (~2?C) was larger than has been recorded elsewhere where the climate is warming, and the effects of increased urbanization or other ‘heat-island’ effects around the recording stations have almost certainly contributed. Temperature records from a recording station in Sakaerat Biosphere Reserve, which lies to the north-east of Khao Yai, at ca 400 m elevation, do not show any increase over the past 35 yr, however (data supplied by Sakaerat Environmental Research Station). Rainfall also showed no consistent pattern among the stations. There was a statistically significant decrease in rainfall at Sakaerat during the years 1969–2004 (mean annual rainfall 1059 mm ± SD 239 mm; r = 0.706; P < 0.0001) and for Prachinburi during 1951–2003 (mean annual rainfall 1929 mm ± SD 297; r = 0.321; P = 0.019). However, the annual rainfall at third site, Pak Chong, to the north of the park, showed no significant trend (mean annual rainfall during 1972–2004: 1113mm±SD 178; r= 0.173; P = 0.337). Temperatures elsewhere in the region (southwestern China) have significantly increased, while rainfall has significantly declined or increased depending on the station. In particular, temperatures most notably increased and rainfall decreased in the lower reaches of the Lancang (Mekong) river (Yunling & Yiping 2005). CONCLUSIONS: FUTURE WORK. - The most plausible explanation is that there has been a real increase in the size and viability of the L. diardi population in the park headquarters area in response to changes in an environmental variable. Lophura nycthemera, in contrast, has not increased, and, allowing for likely increased detectability in both pheasant species, may have actually declined during the study period. The most plausible explanation for the observed trend is climate change. Global climate change may not be the only factor implicated, however. Khao Yai was formerly part of a much larger area of surrounding forest, most of which has since been cleared for farmland. Forest cover in the provinces surrounding Khao Yai was reduced by 25–50 percent during 1973–1995 (Kermel-Torr`es 2004). Fragmentation and associated edge effects such as reported in Pasoh, Malaysia, can negatively affect a wide range of wildlife, including insects, birds, and mammals (Okuda et al. 2003). The more than 2000 km2 area of Khao Yai is a relatively large fragment. However, the impact of large-scale deforestation on regional climate has been demonstrated for Amazonia where, during the dry season, higher temperatures and higher rainfall were observed over deforested areas compared to forested areas (Negri et al. 2004). Loss of forest from areas surrounding Khao Yai might be expected to induce drying and increased temperatures in the forest interior that might also benefit lowland species. Two other species-pairs that might respond similarly to any habitat changes, whose habitat relations are apparently similar to those of the pheasants and whose numbers might be more easily tracked, are orange-breasted trogon Harpactes oreskios (lowland) and red-headed trogon H. erythrocephalus (submontane/montane); and Hainan blue flycatcher Cyornis hainanus (lowland) and hill blue flycatcher C. banyumas (submontane/montane). Both trogons occur on the Mo-Singto permanent plot, in roughly similar numbers (there were 198 orange-breasted trogons detections during May 2003–August 2005 compared with 243 red-headed trogon detections; Khao Yai Avian Diversity Project, unpub. data). They are presumably ecologically segregated, and their co-existence is not surprising, given that as many as four Harpactes spp. may co-exist in lowland Sundaic rain forest (Wells 1999). We have a strong suspicion that C. hainanus has become more common in the past couple of decades. While both C. hainanus and C. banyumas were previously known from the headquarters area of Khao Yai, C. hainanus was usually found in the lower-lying areas, 600–700 m. Only C. banyumas was present on the Mo-Singto Plot during 1982–1983, and again during 2000–2002, when intensive observations were recommenced there. However, at least two C. hainanus appeared on the plot for the first time during 2003. This is too little information on which to base any conclusions, especially as C. hainanus may tend to favor, and move into, small natural clearings and tangles occasioned by treefalls, but it suggests that further monitoring of these species-pairs, in addition to the Lophura pheasants, might be instructive. We cannot yet prove that L. nycthemera has declined as L. diardi has increased in numbers. If climate change is inducing vegetational or other changes that favor the lowland species (L. diardi), this is likely to be at the expense of the upland species, (L. nycthemera) and we should eventually be able to demonstrate an increase in the altitudinal range occupied by L. diardi, and an upslope retreat of L. nycthemera. Up to now, though, there have been few surveys outside of the narrow elevational range around the Khao Yai headquarters in which our studies were conducted. In addition to investigating the detailed ecology and interactions between the two pheasants, future studies should concentrate upon conducting standardized sampling of a wider range of bird species, including, but not limited to, the other species-pairs mentioned, along altitudinal gradients in the park. It would also be necessary to increase the number of meteorological stations in and around the park in order to monitor climatic changes. Most protected areas in the tropics are small fragments of former large expanses of forest. It may take many decades before the full effects of isolation and fragmentation, and edge effects on the communities of plants and animals that livewithin their boundaries, become fully evident. However, the deleterious impacts of these factors are likely to be exacerbated due to climate change. There is an urgent need for more widespread monitoring of population trends among potentially sensitive species, and also for the monitoring of habitat change in tropical forest fragments. |
| ACKNOWLEDGEMENTS We thank the many observers who submitted many of the records on which this paper is based (see also Acknowledgments S1). K. Tokue and K. Pobprasert assisted in compiling records. P. Sanpote, Director, Captive Propagation Division, Department of National Parks (Bangkok), P.Garson, and A.Hennache provided information on captive birds. W. Brockelman, J. W. Duckworth, J. C. Eames, D. Hilbert, T. Savini, D. Wells, and D. Westcott commented on versions of this manuscript. This analysis was conducted while the authors were working on project BRT R_346004 (‘The Khao Yai Avian Diversity Project’) supported by the Biodiversity Research and Training Programme, Thailand. PDR would additionally like to thank The Wetland Trust (UK) for support. |
| REFERENCES Beaumont, L. J., MCALLAN, I. A. W. AND L. HUGHES. (2006). A matter of timing: Changes in the first date of arrival and last date of departure of Australian migratory birds. Global Change Biol. 12: 1339–1354. Birdlife International. (2000). Threatened birds of the world. BirdLife International, Cambridge, UK. Birdlife International. (2001). Threatened birds of Asia: The BirdLife International red data book. BirdLife International, Cambridge, UK. Brockelman, W. Y., Natalang, A., McConkey, K., & Pongsiri, A. (2005). Ecology of the Wild Rambutan Nephelium melliferum Gagnep. [Sapindaceae] in the Mo Singto Forest Dynamics Plot, Khao Yai National Park. Biodiversity research and training program annual meeting, Poster presentation, October 10–13, 2005. Khon Kaen, Thailand. Brockelman, W. Y., Santisuk, T., Nan, C., & Suckaseam, C. (2002). Monitoring plant-animal relations on the Mo-singto Long term biodiversity research plot. Final report to the Biodiversity Research and Training Program, Thailand Research Fund and BIOTEC, NSTDA. Buckland, S. T., Anderson, D. R., Burnham, K. P., Laake, J. L., Borchers, D. L. & Thomas, L. (2001). Introduction to distance sampling: Estimating abundance of biological populations. Oxford University Press, Oxford, UK. Butler, C. J. (2003). The disproportionate effect of global warming on the arrival dates of short-distance migratory birds in North America. Ibis 145: 484–495. Crick, H. Q. P., & Sparks, T. H. (1999). Climate change related to egg-laying trends. Nature 399: 423–424. Crick, H. Q. P., Dudley, C., Glue, D. E. & Thompson, D. L. (1997). UK birds are laying eggs earlier. Nature 388: 526. Davison, G. W. H. (1981). Habitat requirements and the food supply of the Crested Fireback J. World Pheasant Assoc. 6: 40–52. Deignan, H. G. (1963). Checklist of the birds of Thailand. U.S. Nat.Mus. Bull. 226. Smithsonian Institution, Washington, DC. Delacour, J. (1977). The pheasants of the world. 2nd edition. Spur Publications and World Pheasant Association, Reading, UK. Dickinson, E. C. (1963). A preliminary list of the Birds of Khao Yai National Park. Nat. Hist. Bull. Siam Soc. 20: 183–204. Dickinson, E. C. (Ed.). (2003). The Howard &Moore complete checklist of the birds of the world. 3rd edition. Princeton University Press, Princeton, New Jersey. Forcchammer, M., Post, E. & Stenseth, N. C. (1998). Breeding phenology and climate. Nature 391: 29–30. Hickling, R., Roy, D. B., Hill, J. K., Fox, R. & Thomas, C. D. (2006). The distributions of a wide range of taxonomic groups are expanding polewards. Global Change Biol. 12: 450–455. Houghton, J. T., Ding, Y., Griggs, D. J., Nogeur, M., Van Der Linden, P. J. & Xiaosu, D. (2001). Climate change 2001: The scientific basis: Contribution of working group I to the third assessment report of the Intergovernmental Panel on Climate Change (IPCC). Cambridge University Press, Cambridge, UK. Jonzen, N., Linden, A., Ergon, T., Knudsen, E., Vik, J. O., Rubolini, D., Piacentini, D., Brinch, C., Spina, F., Karlsson, L., Stervander, M., Andersson, A., Waldenstrom, J., Lehikoinen, A., Edvardson, E., Solvang, R. & Stenseth. N. C. (2006). Rapid advance of spring arrival dates in long-distance migratory birds. Science 312: 1959–1961. Karr, J. R., & Freemark, K. E. (1983). Habitat selection and environmental gradients: Dynamics in the “stable” tropics. Ecology 64:1481–1494. Kermel-Torres, D. (Ed.) (2004). Atlas of Thailand: Spatial structures and development. IRD Editions, Paris, France. King, B., Dickinson, E. C. & Woodcock, M. W. (1975). A field guide to the birds of South-East Asia. Collins, London, UK. Lehikoinen, E., Sparks, T.H. & Zalakevicius, M. (2004). Arrival and departure dates. In A. P. Møller, W. Fiedler, and P. Berthold (Eds.). Birds and climate change. Advances Ecol. Res. 35. pp. 1–31. Lekagul, B, AND Round, P. D. (1991). A guide to the birds of Thailand. Saha Karn Bhaet Co., Bangkok, Thailand. Lynam, A. J., Round, P.D. & Brockelman, W. Y. (2006). Status of birds and large mammals in Thailand’s Dong Phayayen-Khao Yai Forest Complex.Biodiversity research and training program and Wildlife Conservation Society, Bangkok, Thailand. Malhi, Y. & Wright, J. (2005). Late twentieth-century patterns and trends in the climate of tropical forest regions. In Y. Malhi and O. Phillips (Eds.). Tropical forests and global atmospheric change, pp. 3–16. Oxford University Press, Oxford, UK. Mason, C. F. (1995). Long-term trends in the arrival dates of spring migrants. Bird Study 42: 182–189. McCleery, R. H., & Perrins, C. M. (1998). Temperature and egg-laying trends. Nature 391: 30–31. McClure, H. E. (1974). Some bionomics of the birds of Khao Yai National Park, Thailand. Nat. Hist. Bull. Siam Soc. 25: 99–194. Negri, A. J., Adler, R. F., Xu, L. & Surratt, J. (2004). The impact of Amazonian deforestation on dry season rainfall. J. Climate 17: 1306– 1319. Okuda, T., Suzuki, M., Adachi, N., Yoshida, K., Niiyama, K., Noor, N. S. M., Hussein, N. A., Manokaran, N. & Hashim, M. (2003). Logging history and its impact on forest structure and species composition in the Pasoh Forest Reserve: Implications for the sustainable management of natural resources and landscapes. In T. Okuda, N. Manokaran, Y. Matsumoto, K. Niiyama, S. C. Thomas, and P. S. Ashton (Eds.). Pasoh: ecology of a lowland rain forest in Southeast Asia, pp. 15–34. Springer- Verlag, Tokyo, Japan. Pounds, J. A.,Fogden, M.P.L. & Campbell, J. H. (1999). Biological response to climate change on a tropical mountain. Nature 398: 611–615. Robson, C. (2000). A field guide to the birds of South-East Asia. New Holland, London, UK. Root, T. L., Price, J.T., Hall, K. R., Schneider, S. H., Rosenzweig, C. & Pounds, J. A.. (2003). Fingerprints of global warming on wild animals and plants. Nature 421: 57–60. Schaefer, T., Ledebur, G. Beier, J. & Leisler, B. (2006). Reproductive responses of two related coexisting songbird species to environmental changes: Global warming, competition, and population sizes. J. Ornithol. 147: 47–56. Smitinand, T. (1968). Vegetation of Khao Yai National Park. Nat. His. Bull. Siam Society 22: 289–305. Stibig, H. J., Achard, F. & Fritz, S. (2004). A new forest cover map of southeast Asia derived from SPOT-VEGETTION satellite imagery. Appl. Veg. Sci. 7: 153–162. Thewlis, R.M.,Timmins, R. J., Evans, T.D. & Duckworth, J.W. (1998). The conservation status of birds in Lao PDR. Bird Conserv. Int. 8(suppl.): 1–159. Thomas, C. D., & Lennon, J. J. (1999). Birds extend their ranges northwards. Nature 399: 213. Thomas, L., Laake, J. L., Strindberg, S., Marques, F. F. C., Buckland, S. T., Borchers, D. L., Anderson, D. R., Burnham, K. P., Hedley, S. L., Pollard, J. H. & Bishop, J. R. B. (2004). Distance 5.0. Release Beta 3. Research unit for wildlife population assessment, University of St. Andrews, UK. http://www.ruwpa.st-and.ac.uk/distance/. Wells, D. R. (1985). The forest avifauna of Western Malaysia and its Conservation. In A. W. Diamond and T. E. Lovejoy (Eds.). Conservation of tropical forest birds: Proceedings of a workshop and symposium held at the XVIII World Conference of the International Council for Bird Preservation 7, 8 and 10August 1982 pp. 213–232. ICBPTechnical PublicationNo. 4., International Council for Bird Preservation, Cambridge, UK. Wells, D. R. (1999). The birds of the Thai–Malay Peninsula. Non-Passerines. Vol. 1. Academic Press, London, UK. Yunling, H., & Yiping, Z. (2005). Climate change from 1960 to 2000 in the Lancang River Valley, China. Mountain Res. Develop. 25: 341– 348. |
| Kindly
submitted by: Philip D. Round, Department of Biology, Faculty of Science, Mahidol University, Rama 6 Road, Bangkok 10400, Thailand. Email: frpdr@mahidol.ac.th George A. Gale, King Mongkut’s University of Technology Thonburi, School of Bioresources & Technology, 83 Mu 8 Thakham, Bangkhuntien, Bangkok 10150, Thailand. |
| Tweet |

A Guide to Birdwatching in Thailand. Copyright © 2004-2017 thaibirding.com. All rights reserved.